CROSSFT TFT-55M
GUDID 10845854032633
CROSSFT TAP, 5.5MM
Conmed Corporation
Orthopaedic broach| Primary Device ID | 10845854032633 |
| NIH Device Record Key | aaa0ef4c-2ff9-480e-b525-04f1d9c31e3a |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | CROSSFT |
| Version Model Number | TFT-55M |
| Catalog Number | TFT-55M |
| Company DUNS | 071595540 |
| Company Name | Conmed Corporation |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | true |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | true |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
Device Dimensions
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 10845854032633 [Primary] |
| GS1 | 20845854032630 [Direct Marking] |
FDA Product Code
| HWX | TAP, BONE |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2021-01-27 |
| Device Publish Date | 2021-01-19 |
On-Brand Devices [CROSSFT]
| 20845854045791 | CrossFT Knotless Biocomposite 4.0 mm Suture Anchor with one 2 mm Hi-Fi Tape (Blue) |
| 20845854042660 | CROSSFT 6.5MM SUTURE ANCHOR, W/ 3 HI-FI NO. 2 (5 METRIC) SUTURES W/ NEEDLES |
| 20845854042653 | CROSSFT 6.5MM SUTURE ANCHOR, W/ 2 HI-FI NO. 2 (5 METRIC) SUTURES W/ NEEDLES |
| 20845854042646 | CROSSFT 5.5MM SUTURE ANCHOR, W/ 3 HI-FI NO. 2 (5 METRIC) SUTURES W/ NEEDLES |
| 20845854042639 | CROSSFT 5.5MM SUTURE ANCHOR, W/ 2 HI-FI NO. 2 (5 METRIC) SUTURES W/NEEDLES |
| 20845854042622 | 4.5MM SUTURE ANCHOR, W/ 3 HI-FI NO. 2 (5 METRIC) SUTURES W/ NEEDLES |
| 20845854042615 | CROSSFT 4.5MM SUTURE ANCHOR, W/ 2 HI-FI NO. 2 (5 METRIC) SUTURES W/ NEEDLES |
| 20845854042608 | 6.5MM CROSSFT SUTURE ANCHOR WITH THREE NO. 2 (5 METRIC) HI-FI SUTURES |
| 20845854042592 | 6.5MM CROSSFT SUTURE ANCHOR WITH TWO NO. 2 (5 METRIC) HI-FI SUTURES |
| 20845854042585 | 5.5MM CROSSFT SUTURE ANCHOR WITH THREE NO. 2 (5 METRIC) HI-FI SUTURES |
| 20845854042578 | 5.5MM CROSSFT SUTURE ANCHOR WITH TWO NO. 2 (5 METRIC) HI-FI SUTURES |
| 20845854042561 | 4.5MM CROSSFT SUTURE ANCHOR WITH THREE NO. 2 (5 METRIC) HI-FI SUTURES |
| 20845854042554 | 4.5MM CROSSFT SUTURE ANCHOR WITH TWO NO. 2 (5 METRIC) HI-FI SUTURES |
| 20845854021825 | CROSSFT 6.5MM SUTURE ANCHOR, W/ 3 HI-FI NO. 2 (5 METRIC) SUTURES W/ NEEDLES |
| 20845854021818 | CROSSFT 6.5MM SUTURE ANCHOR, W/ 2 HI-FI NO. 2 (5 METRIC) SUTURES W/ NEEDLES |
| 20845854021801 | CROSSFT 5.5MM SUTURE ANCHOR, W/ 3 HI-FI NO. 2 (5 METRIC) SUTURES W/ NEEDLES |
| 20845854021795 | CROSSFT 5.5MM SUTURE ANCHOR, W/ 2 HI-FI NO. 2 (5 METRIC) SUTURES W/NEEDLES |
| 20845854021788 | CROSSFT 4.5MM SUTURE ANCHOR, W/ 3 HI-FI NO. 2 (5 METRIC) SUTURES W/ NEEDLES |
| 20845854021764 | CROSSFT 4.5MM SUTURE ANCHOR, W/ 2 HI-FI NO. 2 (5 METRIC) SUTURES W/ NEEDLES |
| 20845854014247 | 5.5MM CROSSFT SUTURE ANCHOR WITH THREE NO. 2 (5 METRIC) HI-FI SUTURES |
| 20845854014230 | 4.5MM CROSSFT SUTURE ANCHOR WITH TWO NO. 2 (5 METRIC) HI-FI SUTURES |
| 20845854013981 | 6.5MM CROSSFT SUTURE ANCHOR WITH THREE NO. 2 (5 METRIC) HI-FI SUTURES |
| 20845854013974 | 5.5MM CROSSFT SUTURE ANCHOR WITH TWO NO. 2 (5 METRIC) HI-FI SUTURES |
| 20845854013844 | 4.5MM CROSSFT SUTURE ANCHOR WITH THREE NO. 2 (5 METRIC) HI-FI SUTURES |
| 20845854013530 | 6.5MM CROSSFT SUTURE ANCHOR WITH TWO NO. 2 (5 METRIC) HI-FI SUTURES |
| 20845854028251 | GENESYS CROSSFT PUNCH, 6.5MM |
| 20845854028244 | CROSSFT PUNCH, 6.5MM |
| 20845854028213 | CROSSFT PUNCH, 5.5MM |
| 20845854028190 | GENESYS CROSSFT PUNCH, 4.5MM |
| 20845854028176 | 4.5MM TO 6.5MM ALL IN ONE CROSSFT PUNCH |
| 10845854032657 | CROSSFT TAP, 6.5MM |
| 10845854032633 | CROSSFT TAP, 5.5MM |
| 10845854032619 | CROSSFT TAP, 4.5MM |
| 10845854028186 | CROSSFT PUNCH, 4.5MM |
| 10845854044896 | CrossFT Knotless 5.5 Tap |
| 10845854044889 | CrossFT Knotless 4.75 Tap |
| 10845854044872 | CrossFT Knotless 4.0 Tap |
| 10845854044865 | CrossFT Knotless 5.5 Broaching Punch |
| 10845854044858 | CrossFT Knotless 4.75 Broaching Punch |
| 10845854044841 | CrossFT Knotless 4.0 Broaching Punch |
| 10845854606315 | CrossFT™ Knotless DT 6.5 mm Suture Anchor with #2 Hi-Fi® Suture |
| 10845854606230 | CrossFT™ Knotless DT 6.5 mm Suture Anchor |
| 10845854045107 | CrossFT Knotless 4.75/5.5 Broaching Punch Disposable |
| 10845854045060 | CrossFT Knotless 4.75 Drill Bit, Disposable |
| 10845854045053 | CrossFT Knotless 4.0 Drill Bit, Disposable |
| 10845854045091 | CROSSFT KNOTLESS 4.0 BROACHING PUNCH, DISPOSABLE |
| 10845854045077 | CrossFT Knotless 5.5 Drill Bit, Disposable |
| 10845854044599 | CrossFT Knotless 4.0 mm Suture Anchor with one 2 mm Hi-Fi tape (White/Black) |
| 10845854045800 | CrossFT Knotless Biocomposite 4.0 mm Suture Anchor with one 2 mm Hi-Fi Tape (White/Black) |
| 10845854045862 | CrossFT Knotless Biocomposite 5.5 mm Suture Anchor with one 2 mm Hi-Fi Tape (White/Black) |
Trademark Results [CROSSFT]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
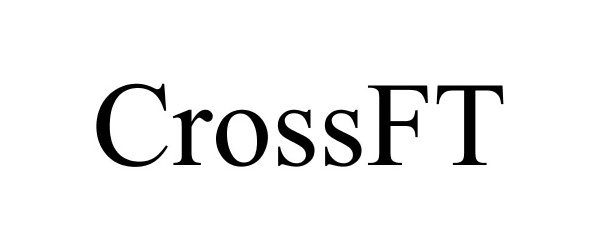 CROSSFT 86920366 5157718 Live/Registered |
Conmed Corporation 2016-02-25 |
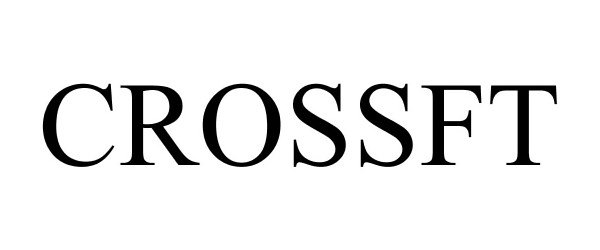 CROSSFT 77720451 not registered Dead/Abandoned |
LINVATEC CORPORATION 2009-04-23 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.