3100 768968
GUDID 10846446018875
MOUNTING BRACKET- SMALL
Carefusion Corporation
Frame/rail/pole device holder| Primary Device ID | 10846446018875 |
| NIH Device Record Key | f5b3cd8b-555e-476d-a9f9-1ff51366fd30 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | 3100 |
| Version Model Number | 768968 |
| Catalog Number | 768968 |
| Company DUNS | 830432451 |
| Company Name | Carefusion Corporation |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | false |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | true |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
| Phone | 800.231.2466 |
| support.vent.us@carefusion.com | |
| Phone | 800.231.2466 |
| support.vent.us@carefusion.com | |
| Phone | 800.231.2466 |
| support.vent.us@carefusion.com | |
| Phone | 800.231.2466 |
| support.vent.us@carefusion.com | |
| Phone | 800.231.2466 |
| support.vent.us@carefusion.com | |
| Phone | 800.231.2466 |
| support.vent.us@carefusion.com | |
| Phone | 800.231.2466 |
| support.vent.us@carefusion.com | |
| Phone | 800.231.2466 |
| support.vent.us@carefusion.com | |
| Phone | 800.231.2466 |
| support.vent.us@carefusion.com | |
| Phone | 800.231.2466 |
| support.vent.us@carefusion.com | |
| Phone | 800.231.2466 |
| support.vent.us@carefusion.com | |
| Phone | 800.231.2466 |
| support.vent.us@carefusion.com | |
| Phone | 800.231.2466 |
| support.vent.us@carefusion.com | |
| Phone | 800.231.2466 |
| support.vent.us@carefusion.com | |
| Phone | 800.231.2466 |
| support.vent.us@carefusion.com | |
| Phone | 800.231.2466 |
| support.vent.us@carefusion.com | |
| Phone | 800.231.2466 |
| support.vent.us@carefusion.com | |
| Phone | 800.231.2466 |
| support.vent.us@carefusion.com | |
| Phone | 800.231.2466 |
| support.vent.us@carefusion.com |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 10846446018875 [Primary] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Approval: P890057
FDA Product Code
| LSZ | Ventilator, High Frequency |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 4 |
| Public Version Date | 2019-06-07 |
| Device Publish Date | 2017-06-16 |
On-Brand Devices [3100]
| 10846446018875 | MOUNTING BRACKET- SMALL |
| 10846446018868 | MTG BRKT HUMIDIFIER |
Trademark Results [3100]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
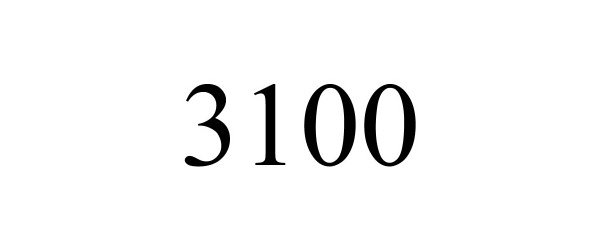 3100 88874131 not registered Live/Pending |
Branch, Jason 2020-04-16 |
 3100 80982539 0982539 Dead/Cancelled |
Xerox Corporation 0000-00-00 |
 3100 80982191 0982191 Dead/Cancelled |
Exerox Corporation 0000-00-00 |
 3100 73833480 1634064 Dead/Cancelled |
GEOCEL CORPORATION 1989-10-23 |
 3100 73270993 1169102 Dead/Cancelled |
Xerox Corporation 1980-07-21 |
 3100 73270971 1184342 Dead/Cancelled |
Xerox Corporation 1980-07-21 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.