Vmax 777494
GUDID 10846446021110
VMAX ENCORE 22 UNIT ASSEMBLY
Carefusion Corporation
Pulmonary function analysis system, paediatric| Primary Device ID | 10846446021110 |
| NIH Device Record Key | f5a6f39c-a72a-4d53-8015-ec56693f78c8 |
| Commercial Distribution Discontinuation | 2022-03-31 |
| Commercial Distribution Status | Not in Commercial Distribution |
| Brand Name | Vmax |
| Version Model Number | 777494 |
| Catalog Number | 777494 |
| Company DUNS | 830432451 |
| Company Name | Carefusion Corporation |
| Device Count | 1 |
| DM Exempt | true |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | false |
| Serial Number | true |
| Manufacturing Date | true |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
| Phone | 800.231.2466 |
| support.vmax.us@carefusion.com | |
| Phone | 800.231.2466 |
| support.vmax.us@carefusion.com | |
| Phone | 800.231.2466 |
| support.vmax.us@carefusion.com | |
| Phone | 800.231.2466 |
| support.vmax.us@carefusion.com | |
| Phone | 800.231.2466 |
| support.vmax.us@carefusion.com | |
| Phone | 800.231.2466 |
| support.vmax.us@carefusion.com | |
| Phone | 800.231.2466 |
| support.vmax.us@carefusion.com | |
| Phone | 800.231.2466 |
| support.vmax.us@carefusion.com | |
| Phone | 800.231.2466 |
| support.vmax.us@carefusion.com | |
| Phone | 800.231.2466 |
| support.vmax.us@carefusion.com | |
| Phone | 800.231.2466 |
| support.vmax.us@carefusion.com | |
| Phone | 800.231.2466 |
| support.vmax.us@carefusion.com | |
| Phone | 800.231.2466 |
| support.vmax.us@carefusion.com | |
| Phone | 800.231.2466 |
| support.vmax.us@carefusion.com | |
| Phone | 800.231.2466 |
| support.vmax.us@carefusion.com | |
| Phone | 800.231.2466 |
| support.vmax.us@carefusion.com |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 10846446021110 [Primary] |
FDA Product Code
| BTY | Calculator, Predicted Values, Pulmonary Function |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 3 |
| Public Version Date | 2024-06-06 |
| Device Publish Date | 2016-09-16 |
On-Brand Devices [Vmax]
| 10846446026443 | OPTIPLEX XE2 WIN7 64BIT ENGLISH 100_240V (VMAX) |
| 10846446032703 | KIT,DUAL ARM DUAL MONITOR,OPTIPLEX XE |
| 10846446032383 | SWR KIT,VMAX TO SENTRY SUITE CONNECTIVITY |
| 10846446027716 | SWR KIT,DATA CONVERT - VMAX TO SQL |
| 10846446027693 | SOFTWARE KIT VMAX ENCORE VER 21-1 |
| 10846446027624 | SWR KIT VMAX ENCORE VER 20-7 |
| 10846446027600 | SOFTWARE KIT,VMAX ENCORE VERSION 20-5B CD |
| 10846446020441 | SWR KIT VIASYS SECURITY ADMIN 4.65.6.0 |
| 10846446020298 | SWR KIT,VMAX ENCORE VER 21-2B |
| 10846446020281 | SWR KIT VMAX SPECTRA VER 12-7 |
| 10846446020793 | ECG INTEGRATED 3-LEAD |
| 10846446033045 | SNIP & MAX PRESSURE OPTION ENCORE |
| 10846446033038 | AUTOBOX ASSY WHEELCHAIR V62J REFURBISHED |
| 10846446033021 | AUTOBOX ASSY STANDARD V62J REFURBISHED |
| 10846446032987 | SWR KIT VMAX ENCORE VER 28-5 SERIES |
| 10846446032949 | VMAX READING STATION UPGRADE |
| 10846446032925 | VMAX SOFTWARE UPGRADE |
| 10846446032918 | SWR KIT CARDIOSOFT V6.73 |
| 10846446032901 | SWR PKG CARDIOSOFT V6.73 |
| 10846446032888 | CARDIOSOFT V6.73 CAM14 W/USB |
| 10846446032840 | KIT,SWR VMAX ENCORE 21-2D |
| 10846446032833 | KIT SWR VMAX ENCORE 27-3D |
| 10846446032826 | SINGLE MONITOR CONSOLE |
| 10846446032819 | DUAL HORIZONTAL MONITOR CONSOLE |
| 10846446032802 | DUAL STACKED MONITOR CONSOLE |
| 10846446032734 | CD,VMAX VER 28 |
| 10846446032697 | VMAX ENCORE 29C,REFURB |
| 10846446032680 | VMAX ENCORE 29,REFURB |
| 10846446032673 | VMAX ENCORE 22D,REFURB |
| 10846446032666 | VMAX ENCORE 229D,REFURB |
| 10846446032659 | VMAX ENCORE 229,REFURB |
| 10846446032642 | VMAX ENCORE 20C UNIT ASSEMBLY |
| 10846446030358 | VMAX ENCORE 229C UNIT ASSEMBLY |
| 10846446030341 | VMAX ENCORE 29C UNIT ASSEMBLY |
| 10846446030334 | VMAX ENCORE 29 UNIT ASSEMBLY |
| 10846446030327 | VMAX ENCORE 229 UNIT ASSEMBLY |
| 10846446030228 | VMAX ENCORE 229C,REFURB |
| 10846446030143 | VMAX ENCORE 22,REFURB |
| 10846446030136 | VMAX ENCORE 229D |
| 10846446029642 | LC CART ASSY W/CBLS VMAX DOM |
| 10846446029178 | AUTOBOX ASSY WHEELCHAIR V62J |
| 10846446029109 | AUTOBOX ASSEMBLY,STANDARD V62J |
| 10846446026467 | VMAX SECURITY ADMIN KIT |
| 10846446026139 | VMAX ENCORE 229C UNIT ASSEMBLY (Refurb) |
| 10846446026122 | VMAX ENCORE 29C UNIT ASSEMBLY (Refurb) |
| 10846446026115 | VMAX ENCORE 29 UNIT ASSEMBLY (Refurb) |
| 10846446026092 | VMAX ENCORE 22 UNIT ASSEMBLY (Refurb) |
| 10846446026085 | VMAX ENCORE 229 UNIT ASSEMBLY (Refurb) |
| 10846446025620 | VMAX ENC/SES SMALL SYS SERVER SW |
| 10846446025583 | READING STATION VMAX/SES 2ND AND SUBS |
Trademark Results [Vmax]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 VMAX 98521801 not registered Live/Pending |
Hong Kong IVPS International Limited 2024-04-26 |
 VMAX 90057375 not registered Live/Pending |
VMAX Biotechnology Inc. 2020-07-16 |
 VMAX 87113298 5164870 Live/Registered |
Kaltec Electronics, Inc. 2016-07-22 |
 VMAX 86813549 5142893 Live/Registered |
Flight Training Centers of America LLC 2015-11-09 |
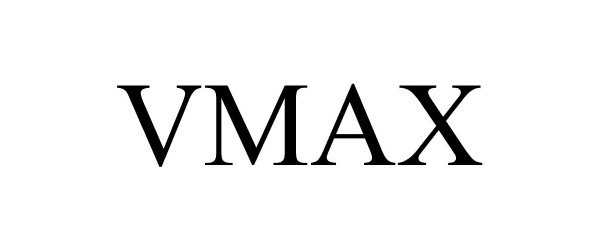 VMAX 86306930 4869151 Live/Registered |
NY Thermal Inc. 2014-06-11 |
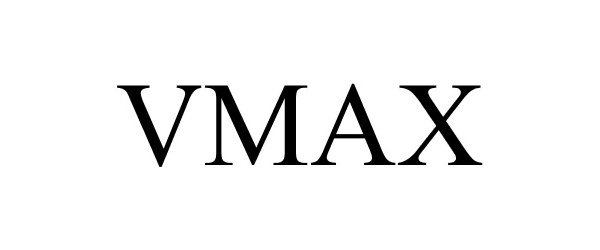 VMAX 86163674 4580023 Live/Registered |
FORUM US, INC. 2014-01-13 |
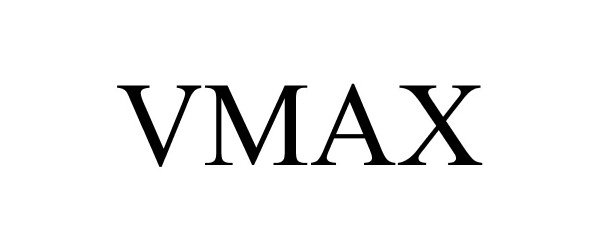 VMAX 86156955 not registered Dead/Abandoned |
CPI Sales & Manufacturing, Inc. 2014-01-03 |
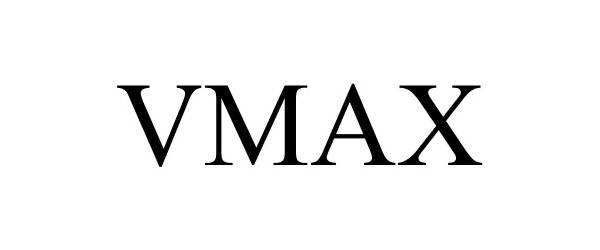 VMAX 85840350 4625575 Live/Registered |
World Dryer Corporation 2013-02-04 |
 VMAX 85500716 4335815 Live/Registered |
WESTERN GREEN, LLC 2011-12-21 |
 VMAX 79336141 not registered Live/Pending |
Vmax Global AG 2021-12-23 |
 VMAX 79277452 not registered Live/Pending |
YAMAHA HATSUDOKI KABUSHIKI KAISHA 2019-12-03 |
 VMAX 78340767 2999099 Dead/Cancelled |
VIALOGY, LLC 2003-12-15 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.