Hexa CU-2009
GUDID 10850023750751
Hexa Composite Enamel BLEACH, Caplets (20)
Hexa Dental
Dental composite resin kit| Primary Device ID | 10850023750751 |
| NIH Device Record Key | c9b2a6e6-47ce-4346-861c-ad218c852d89 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | Hexa |
| Version Model Number | CU-2009 |
| Catalog Number | CU-2009 |
| Company DUNS | 096955608 |
| Company Name | Hexa Dental |
| Device Count | 20 |
| DM Exempt | false |
| Pre-market Exempt | true |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | true |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | false |
| OTC Over-The-Counter | false |
Customer Support Contacts
| Phone | +1(626)677-0486 |
| faraz@hygedentusa.com | |
| Phone | +1(626)677-0486 |
| faraz@hygedentusa.com | |
| Phone | +1(626)677-0486 |
| faraz@hygedentusa.com | |
| Phone | +1(626)677-0486 |
| faraz@hygedentusa.com | |
| Phone | +1(626)677-0486 |
| faraz@hygedentusa.com | |
| Phone | +1(626)677-0486 |
| faraz@hygedentusa.com | |
| Phone | +1(626)677-0486 |
| faraz@hygedentusa.com | |
| Phone | +1(626)677-0486 |
| faraz@hygedentusa.com | |
| Phone | +1(626)677-0486 |
| faraz@hygedentusa.com | |
| Phone | +1(626)677-0486 |
| faraz@hygedentusa.com | |
| Phone | +1(626)677-0486 |
| faraz@hygedentusa.com | |
| Phone | +1(626)677-0486 |
| faraz@hygedentusa.com | |
| Phone | +1(626)677-0486 |
| faraz@hygedentusa.com | |
| Phone | +1(626)677-0486 |
| faraz@hygedentusa.com | |
| Phone | +1(626)677-0486 |
| faraz@hygedentusa.com | |
| Phone | +1(626)677-0486 |
| faraz@hygedentusa.com | |
| Phone | +1(626)677-0486 |
| faraz@hygedentusa.com |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00850023750754 [Unit of Use] |
| GS1 | 10850023750751 [Primary] |
| GS1 | 20850023750758 [Package] Package: box [64 Units] In Commercial Distribution |
FDA Product Code
| EBF | Material, tooth shade, resin |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 3 |
| Public Version Date | 2024-01-11 |
| Device Publish Date | 2023-03-28 |
On-Brand Devices [Hexa]
| 00850023750235 | Bite registration |
| 00850023750228 | Temp Crown And Bridge Shade:B1 |
| 00850023750211 | Temp Crown And Bridge Shade:A3 |
| 00850023750204 | Temp Crown And Bridge Shade:A2 |
| 00850023750198 | Temp Crown And Bridge Shade:A1 |
| 00850023750181 | Flowable Composite |
| 00850023750174 | Flowable Composite |
| 00850023750167 | Flowable Composite |
| 00850023750150 | Flowable Composite |
| 00850023750143 | Flowable Composite |
| 00850023750136 | Bonding Agent |
| 00850023750129 | PGA Undyed Suture |
| 00850023750112 | PGA Undyed Suture |
| 00850023750105 | PGA Violet Suture |
| 00850023750099 | PGA Violet Suture |
| 00850023750082 | PGA Violet Suture |
| 00850023750075 | Silk Suture |
| 00850023750068 | Silk Suture |
| 00850023750051 | Silk Suture |
| 00850023750044 | Silk Suture |
| 00850023750037 | Silk Suture |
| 00850023750013 | Nylon Suture |
| 00850023750006 | DentalEtching Get |
| 10850023750348 | Hexa 5% Sodium Fluoride Varnish Assorted Flavors . 5ml 50/pk |
| 10850023750331 | Hexa Prophy Paste Splatter-Free Containing 1.23% Fluoride (Coarse) Assorted, 200/Pk |
| 10850023750324 | Hexa Prophy Paste Splatter-Free Containing 1.23% Fluoride (Coarse) Mint , 200/Pk |
| 10850023750317 | Hexa Prophy Paste Splatter-Free Containing 1.23% Fluoride (Coarse) Cherry , 200/Pk |
| 10850023750300 | Hexa Prophy Paste Splatter-Free Containing 1.23% Fluoride (Coarse) Bubblegum , 200/Pk |
| 10850023750294 | Hexa Prophy Paste Splatter-Free Containing 1.23% Fluoride (Medium) Assorted , 200/Pk |
| 10850023750287 | Hexa Prophy Paste Splatter-Free Containing 1.23% Fluoride (Medium) Mint , 200/Pk |
| 10850023750270 | Hexa Prophy Paste Splatter-Free Containing 1.23% Fluoride (Medium) Cherryl , 200/Pk |
| 10850023750263 | Hexa Prophy Paste Splatter-Free Containing 1.23% Fluoride (Medium) Bubblegum , 200/Pk |
| 10850023750256 | Hexa Prophy Paste Splatter-Free Containing 1.23% Fluoride (Fine) Bubblegum , 200/Pk |
| 00850057092134 | Hexa Semi Permanent Implant Cement |
| 00850057092127 | Hexa Non-Eugenol Temporary Cement |
| 00850057092110 | Hexa Pit And Fissure Sealant |
| 00850057092103 | HexALGIN, Chromatic Alginate Dental Impression Material , Berry Flavor |
| 00850057092097 | HexALGIN, Chromatic Alginate Dental Impression Material , Mint Flavor |
| 00850057092080 | Hexa Disposable Stainless Steel Scalpels #15C |
| 00850057092073 | Hexa Disposable Stainless Steel Scalpels #25 |
| 00850057092066 | Hexa Disposable Stainless Steel Scalpels #21 |
| 00850057092059 | Hexa Disposable Stainless Steel Scalpels #15 |
| 00850057092042 | Hexa Disposable Stainless Steel Scalpels #12 |
| 00850057092035 | Hexa Disposable Stainless Steel Scalpels #11 |
| 00850057092028 | Hexa Surgical Blade #25 Stainless Steel (100) |
| 00850057092011 | Hexa Surgical Blade #21 Stainless Steel |
| 00850057092004 | Hexa Surgical Blade #15C Stainless Steel |
| 00850023750990 | Hexa Surgical Blade #15 Stainless Steel (100) |
| 00850023750983 | Hexa Surgical Blade #12D Stainless Steel (100) |
| 00850023750976 | Hexa Surgical Blade #12 Stainless Steel (100) |
Trademark Results [Hexa]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
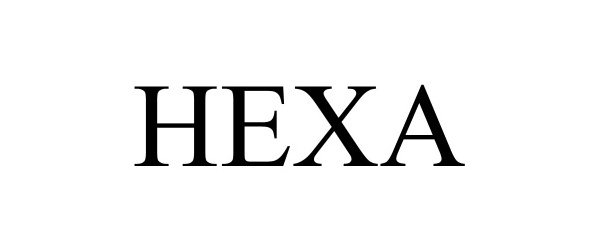 HEXA 98529483 not registered Live/Pending |
HEXA USA LLC 2024-05-01 |
 HEXA 98396955 not registered Live/Pending |
HEXA CO., LTD. 2024-02-07 |
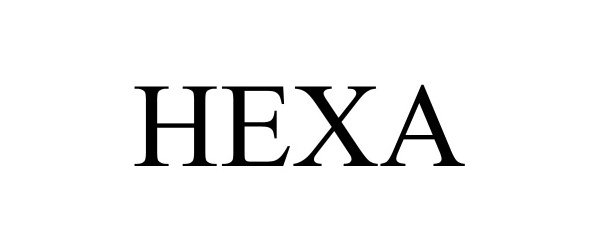 HEXA 98223986 not registered Live/Pending |
Techo-Bloc inc. 2023-10-14 |
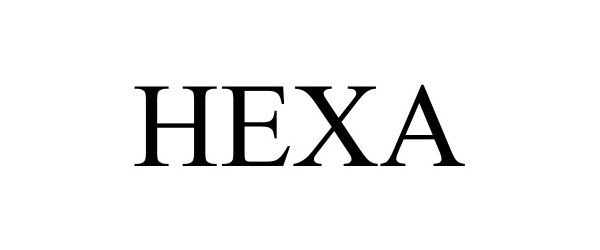 HEXA 97891740 not registered Live/Pending |
Humeiq Inc. 2023-04-17 |
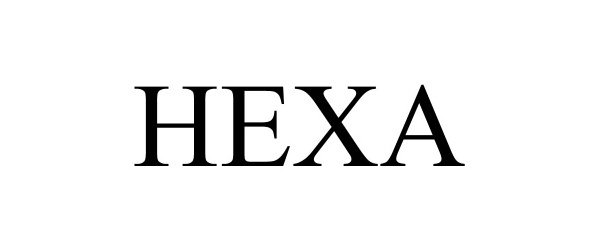 HEXA 90775478 not registered Live/Pending |
Techzillion LLC 2021-06-15 |
 HEXA 90441290 not registered Live/Pending |
Hygedent USA 2020-12-31 |
 HEXA 88979836 not registered Live/Pending |
Spectrum Diversified Designs, LLC 2020-03-26 |
 HEXA 88848366 not registered Live/Pending |
Spectrum Diversified Designs, LLC 2020-03-26 |
 HEXA 87862219 not registered Live/Pending |
Orbs Ltd. 2018-04-04 |
 HEXA 87862213 not registered Live/Pending |
Orbs Ltd. 2018-04-04 |
 HEXA 87579307 5596973 Live/Registered |
Hygedent USA 2017-08-22 |
 HEXA 87576736 not registered Live/Pending |
Interactiv Corporation 2017-08-21 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.