True Form™ TF14165S/A
GUDID 10884450297879
Merit Medical Systems, Inc.
Cardiac/peripheral vascular guidewire, single-use| Primary Device ID | 10884450297879 |
| NIH Device Record Key | 7a658711-04ca-424d-8aa2-4304c899fd9b |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | True Form™ |
| Version Model Number | 00884450297872 |
| Catalog Number | TF14165S/A |
| Company DUNS | 184763290 |
| Company Name | Merit Medical Systems, Inc. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | false |
| OTC Over-The-Counter | false |
Device Dimensions
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00884450297872 [Primary] |
| GS1 | 10884450297879 [Package] Contains: 00884450297872 Package: [5 Units] In Commercial Distribution |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K170532
FDA Product Code
| DQX | Wire, guide, catheter |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | true |
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 4 |
| Public Version Date | 2019-03-22 |
| Device Publish Date | 2017-10-16 |
On-Brand Devices [True Form™]
| 10884450297893 | 00884450297896 |
| 10884450297886 | 00884450297889 |
| 10884450297879 | 00884450297872 |
| 10884450297862 | 00884450297865 |
| 10884450297848 | 00884450297841 |
| 10884450329761 | 00884450329764 |
Trademark Results [True Form]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 TRUE FORM 98700618 not registered Live/Pending |
Vision LLC 2024-08-15 |
 TRUE FORM 90236621 not registered Live/Pending |
Express Recycling & Collections Inc 2020-10-05 |
 TRUE FORM 87420855 5499097 Live/Registered |
Merit Medical Systems, Inc. 2017-04-21 |
 TRUE FORM 85533441 4332727 Dead/Cancelled |
TRUE FORM, LLC 2012-02-03 |
 TRUE FORM 79234913 not registered Dead/Abandoned |
LARY S.a.r.l. 2018-05-18 |
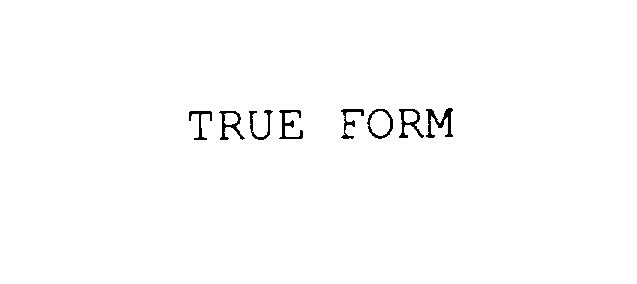 TRUE FORM 78018556 not registered Dead/Abandoned |
Ball Screws & Actuators Co., Inc. 2000-07-27 |
 TRUE FORM 73804175 1577556 Dead/Cancelled |
ADOBE SYSTEMS INCORPORATED 1989-06-01 |
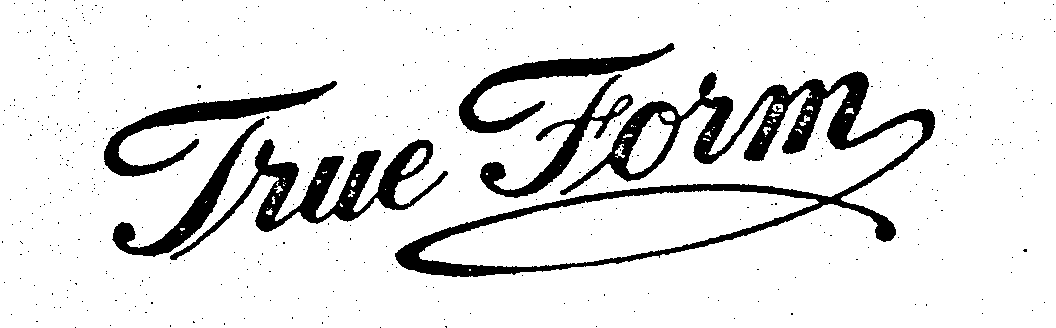 TRUE FORM 71188871 0186657 Dead/Cancelled |
True Form Corset Co. 1923-11-24 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.