StabiliT® 1436-01/B
GUDID 10884450315924
Merit Medical Systems, Inc.
Orthopaedic cement preparation/delivery kit| Primary Device ID | 10884450315924 |
| NIH Device Record Key | b98dc11b-8ae4-49ae-8a36-c7336ec457eb |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | StabiliT® |
| Version Model Number | 00884450315927 |
| Catalog Number | 1436-01/B |
| Company DUNS | 184763290 |
| Company Name | Merit Medical Systems, Inc. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | false |
| OTC Over-The-Counter | false |
Device Dimensions
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00884450315927 [Primary] |
| GS1 | 10884450315924 [Package] Contains: 00884450315927 Package: [1 Units] In Commercial Distribution |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K072496
FDA Product Code
| NDN | CEMENT, BONE, VERTEBROPLASTY |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 2 |
| Public Version Date | 2018-07-06 |
| Device Publish Date | 2018-04-10 |
On-Brand Devices [StabiliT®]
| 10884450379858 | 00884450379851 |
| 10884450379841 | 00884450379844 |
| 10884450379834 | 00884450379837 |
| 10884450379827 | 00884450379820 |
| 00884450374344 | 00884450374344 |
| 10884450325763 | 00884450325766 |
| 10884450325701 | 00884450325704 |
| 10884450325664 | 00884450325667 |
| 10884450325640 | 00884450325643 |
| 10884450325619 | 00884450325612 |
| 10884450325602 | 00884450325605 |
| 10884450317669 | 00884450317662 |
| 10884450317652 | 00884450317655 |
| 10884450317645 | 00884450317648 |
| 10884450317638 | 00884450317631 |
| 10884450315924 | 00884450315927 |
| 10884450315917 | 00884450315910 |
| 10884450315900 | 00884450315903 |
| 10884450315894 | 00884450315897 |
| 10884450314484 | 00884450314487 |
| 10884450314316 | 00884450314319 |
| 10884450296506 | 00884450296509 |
| 10884450296469 | 00884450296462 |
| 10884450296414 | 00884450296417 |
| 10884450296308 | 00884450296301 |
| 10884450296278 | 00884450296271 |
| 10884450296247 | 00884450296240 |
| 10884450296223 | 00884450296226 |
| 10884450296216 | 00884450296219 |
| 00884450374337 | 00884450374337 |
| 10884450379902 | 00884450379905 |
| 00884450314074 | 00884450314074 |
| 10884450390457 | 00884450390450 |
| 10884450413996 | 00884450413999 |
| 00884450314081 | 00884450314081 |
| 00884450469057 | 00884450469057 |
| 10884450441456 | 00884450441459 |
| 10884450390471 | 00884450390474 |
| 10884450390464 | 00884450390467 |
| 00884450469064 | 00884450469064 |
| 10884450503338 | 00884450503331 |
| 10884450390440 | 00884450390443 |
| 10884450492366 | 00884450492369 |
| 10884450296230 | 00884450296233 |
| 00884450469088 | 00884450469088 |
| 00884450492444 | 00884450492444 |
| 00884450492437 | 10884450492434 |
| 00884450492352 | 10884450492359 |
| 00884450492420 | 00884450492420 |
| 10884450492373 | 00884450492376 |
Trademark Results [StabiliT]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
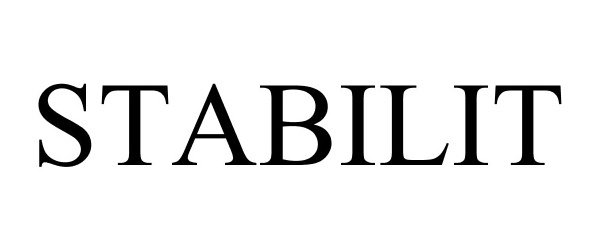 STABILIT 88227104 not registered Live/Pending |
Stabilit Servicios, S.A. de C.V. 2018-12-12 |
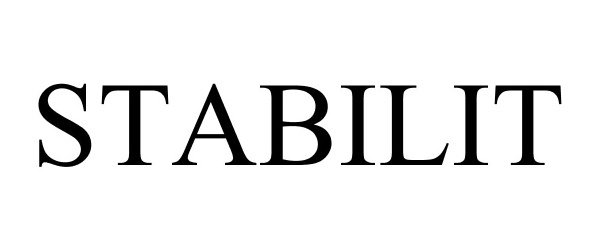 STABILIT 88073432 not registered Live/Pending |
STABILIT SERVICIOS, S.A. DE C.V. 2018-08-10 |
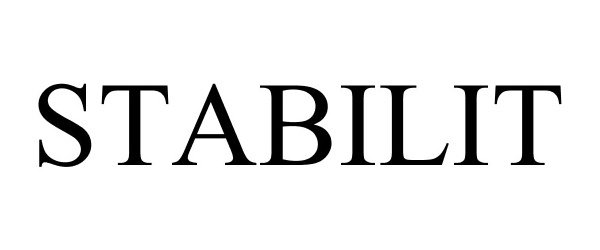 STABILIT 85175465 3978157 Dead/Cancelled |
STABILIT SERVICIOS, S.A. DE C.V. 2010-11-12 |
 STABILIT 78216153 2795049 Dead/Cancelled |
STABILIT SERVICIOS, S.A. DE C.V. 2003-02-18 |
 STABILIT 77421445 3712775 Live/Registered |
DFine, Inc. 2008-03-13 |
 STABILIT 74665386 1983821 Dead/Cancelled |
STABILIT, S.A. DE C.V. 1995-04-24 |
 STABILIT 74406992 not registered Dead/Abandoned |
Stabilit, S.A. de C.V 1993-06-25 |
 STABILIT 74406989 not registered Dead/Abandoned |
Stabilit, S.A. de C.V 1993-06-25 |
 STABILIT 73175943 1129756 Dead/Cancelled |
HENKEL KOMMADITGESELLSCHAFT AUF AKTIEN(HENKEL KGAA) 1978-06-26 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.