Polysorb 160023
GUDID 10884521176966
Obturator
Covidien LP
Tendon/ligament bone anchor, bioabsorbable| Primary Device ID | 10884521176966 |
| NIH Device Record Key | d06a8258-465f-4036-99d8-76d824d5e52f |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | Polysorb |
| Version Model Number | 160023 |
| Catalog Number | 160023 |
| Company DUNS | 058614483 |
| Company Name | Covidien LP |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
| Phone | +1(508)261-8000 |
| covidien.udi@covidien.com | |
| Phone | +1(508)261-8000 |
| covidien.udi@covidien.com | |
| Phone | +1(508)261-8000 |
| covidien.udi@covidien.com | |
| Phone | +1(508)261-8000 |
| covidien.udi@covidien.com | |
| Phone | +1(508)261-8000 |
| covidien.udi@covidien.com | |
| Phone | +1(508)261-8000 |
| covidien.udi@covidien.com | |
| Phone | +1(508)261-8000 |
| covidien.udi@covidien.com | |
| Phone | +1(508)261-8000 |
| covidien.udi@covidien.com | |
| Phone | +1(508)261-8000 |
| covidien.udi@covidien.com | |
| Phone | +1(508)261-8000 |
| covidien.udi@covidien.com | |
| Phone | +1(508)261-8000 |
| covidien.udi@covidien.com | |
| Phone | +1(508)261-8000 |
| covidien.udi@covidien.com | |
| Phone | +1(508)261-8000 |
| covidien.udi@covidien.com | |
| Phone | +1(508)261-8000 |
| covidien.udi@covidien.com | |
| Phone | +1(508)261-8000 |
| covidien.udi@covidien.com | |
| Phone | +1(508)261-8000 |
| covidien.udi@covidien.com | |
| Phone | +1(508)261-8000 |
| covidien.udi@covidien.com | |
| Phone | +1(508)261-8000 |
| covidien.udi@covidien.com | |
| Phone | +1(508)261-8000 |
| covidien.udi@covidien.com | |
| Phone | +1(508)261-8000 |
| covidien.udi@covidien.com | |
| Phone | +1(508)261-8000 |
| covidien.udi@covidien.com | |
| Phone | +1(508)261-8000 |
| covidien.udi@covidien.com | |
| Phone | +1(508)261-8000 |
| covidien.udi@covidien.com | |
| Phone | +1(508)261-8000 |
| covidien.udi@covidien.com | |
| Phone | +1(508)261-8000 |
| covidien.udi@covidien.com | |
| Phone | +1(508)261-8000 |
| covidien.udi@covidien.com | |
| Phone | +1(508)261-8000 |
| covidien.udi@covidien.com | |
| Phone | +1(508)261-8000 |
| covidien.udi@covidien.com | |
| Phone | +1(508)261-8000 |
| covidien.udi@covidien.com | |
| Phone | +1(508)261-8000 |
| covidien.udi@covidien.com | |
| Phone | +1(508)261-8000 |
| covidien.udi@covidien.com | |
| Phone | +1(508)261-8000 |
| covidien.udi@covidien.com | |
| Phone | +1(508)261-8000 |
| covidien.udi@covidien.com | |
| Phone | +1(508)261-8000 |
| covidien.udi@covidien.com | |
| Phone | +1(508)261-8000 |
| covidien.udi@covidien.com | |
| Phone | +1(508)261-8000 |
| covidien.udi@covidien.com | |
| Phone | +1(508)261-8000 |
| covidien.udi@covidien.com | |
| Phone | +1(508)261-8000 |
| covidien.udi@covidien.com | |
| Phone | +1(508)261-8000 |
| covidien.udi@covidien.com | |
| Phone | +1(508)261-8000 |
| covidien.udi@covidien.com | |
| Phone | +1(508)261-8000 |
| covidien.udi@covidien.com | |
| Phone | +1(508)261-8000 |
| covidien.udi@covidien.com | |
| Phone | +1(508)261-8000 |
| covidien.udi@covidien.com | |
| Phone | +1(508)261-8000 |
| covidien.udi@covidien.com | |
| Phone | +1(508)261-8000 |
| covidien.udi@covidien.com | |
| Phone | +1(508)261-8000 |
| covidien.udi@covidien.com | |
| Phone | +1(508)261-8000 |
| covidien.udi@covidien.com | |
| Phone | +1(508)261-8000 |
| covidien.udi@covidien.com |
Device Dimensions
| Outer Diameter | 3 Millimeter |
| Outer Diameter | 3 Millimeter |
| Outer Diameter | 3 Millimeter |
| Outer Diameter | 3 Millimeter |
| Outer Diameter | 3 Millimeter |
| Outer Diameter | 3 Millimeter |
| Outer Diameter | 3 Millimeter |
| Outer Diameter | 3 Millimeter |
| Outer Diameter | 3 Millimeter |
| Outer Diameter | 3 Millimeter |
| Outer Diameter | 3 Millimeter |
| Outer Diameter | 3 Millimeter |
| Outer Diameter | 3 Millimeter |
| Outer Diameter | 3 Millimeter |
| Outer Diameter | 3 Millimeter |
| Outer Diameter | 3 Millimeter |
| Outer Diameter | 3 Millimeter |
| Outer Diameter | 3 Millimeter |
| Outer Diameter | 3 Millimeter |
| Outer Diameter | 3 Millimeter |
| Outer Diameter | 3 Millimeter |
| Outer Diameter | 3 Millimeter |
| Outer Diameter | 3 Millimeter |
| Outer Diameter | 3 Millimeter |
| Outer Diameter | 3 Millimeter |
| Outer Diameter | 3 Millimeter |
| Outer Diameter | 3 Millimeter |
| Outer Diameter | 3 Millimeter |
| Outer Diameter | 3 Millimeter |
| Outer Diameter | 3 Millimeter |
| Outer Diameter | 3 Millimeter |
| Outer Diameter | 3 Millimeter |
| Outer Diameter | 3 Millimeter |
| Outer Diameter | 3 Millimeter |
| Outer Diameter | 3 Millimeter |
| Outer Diameter | 3 Millimeter |
| Outer Diameter | 3 Millimeter |
| Outer Diameter | 3 Millimeter |
| Outer Diameter | 3 Millimeter |
| Outer Diameter | 3 Millimeter |
| Outer Diameter | 3 Millimeter |
| Outer Diameter | 3 Millimeter |
| Outer Diameter | 3 Millimeter |
| Outer Diameter | 3 Millimeter |
| Outer Diameter | 3 Millimeter |
| Outer Diameter | 3 Millimeter |
| Outer Diameter | 3 Millimeter |
Operating and Storage Conditions
| Special Storage Condition, Specify | Between 0 and 0 *; |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 10884521176966 [Primary] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K961578
FDA Product Code
| JDR | STAPLE, FIXATION, BONE |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 6 |
| Public Version Date | 2019-04-22 |
| Device Publish Date | 2015-08-28 |
On-Brand Devices [Polysorb]
| 10884521049543 | Braided Absorbable Suture |
| 10884521048478 | Braided Absorbable Suture |
| 10884521042629 | Braided Absorbable Suture |
| 10884521176836 | Meniscal Stapler Up Curved Loading Unit |
| 10884521176829 | Meniscal Stapler Straight Loading Unit |
| 10884521126831 | Absorbable Single Stitch Reload |
| 10884521126824 | Absorbable Single Stitch Reload |
| 10884521126817 | Absorbable Single Stitch Reload |
| 10884521126800 | Absorbable Single Stitch Reload |
| 10884521126794 | Absorbable Single Stitch Reload |
| 10884521126787 | Absorbable Single Stitch Reload |
| 10884521126770 | Absorbable Single Stitch Reload |
| 10884521126763 | Absorbable Single Stitch Reload |
| 10884521126756 | Absorbable Single Stitch Reload |
| 10884521126749 | Absorbable Single Stitch Reload |
| 10884521126732 | Absorbable Single Stitch Reload |
| 10884521126619 | Absorbable Single Stitch Reload |
| 10884521126602 | Absorbable Single Stitch Reload |
| 10884521126596 | Absorbable Single Stitch Reload |
| 10884521126589 | Absorbable Single Stitch Reload |
| 10884521126572 | Absorbable Single Stitch Reload |
| 20884521133577 | Braided Absorbable Suture |
| 20884521130767 | Braided Absorbable Suture |
| 20884521130750 | Braided Absorbable Suture |
| 20884521130712 | Braided Absorbable Suture |
| 20884521130682 | Braided Absorbable Suture |
| 20884521130675 | Braided Absorbable Suture |
| 20884521046211 | Braided Absorbable Suture |
| 20884521045474 | Braided Absorbable Suture |
| 10884521181625 | Bone Punch |
| 10884521176966 | Obturator |
| 10884521176942 | Twist Drill bit |
| 10884521176935 | Drill Bit |
| 10884521176904 | Drill Guide |
| 20884521184050 | Braided Absorbable Suture |
| 20884521048840 | Braided Absorbable Suture |
| 20884521046952 | Braided Absorbable Suture |
| 20884521043760 | Braided Absorbable Suture |
| 20884521139555 | Braided Absorbable Suture |
| 20884521135137 | Braided Absorbable Suture |
| 20884521130781 | Braided Absorbable Suture |
| 20884521130743 | Braided Absorbable Suture |
| 20884521130606 | Braided Absorbable Suture |
| 20884521120157 | Braided Absorbable Suture |
| 20884521101743 | Braided Absorbable Suture |
| 20884521044255 | Braided Absorbable Suture |
| 20884521043562 | Braided Absorbable Suture |
| 20884521043517 | Braided Absorbable Suture |
| 20884521042725 | Braided Absorbable Suture |
| 20884521042664 | Braided Absorbable Suture |
Trademark Results [Polysorb]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
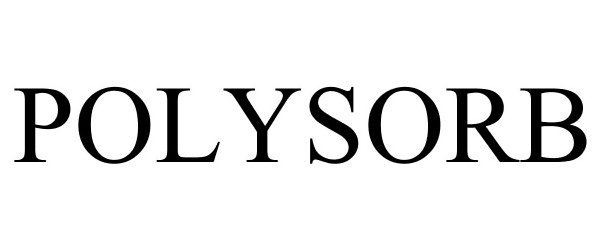 POLYSORB 87701132 not registered Dead/Abandoned |
Gypsorb LLC 2017-11-29 |
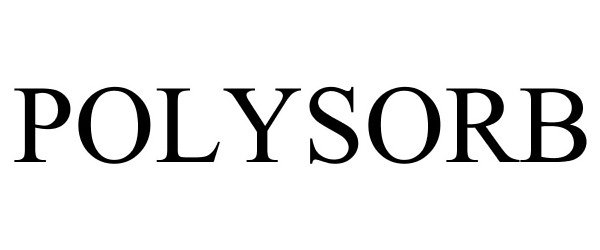 POLYSORB 86409827 4833424 Live/Registered |
Multisorb Technologies, Inc. 2014-09-30 |
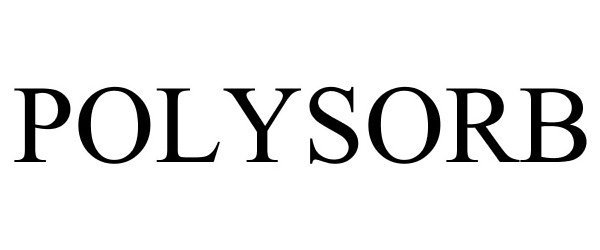 POLYSORB 85410540 4102572 Live/Registered |
COVIDIEN LP 2011-08-30 |
 POLYSORB 80981847 0981847 Dead/Cancelled |
E. Fougera & Co., Inc. 0000-00-00 |
 POLYSORB 78290719 not registered Dead/Abandoned |
Johns Manville International, Inc. 2003-08-21 |
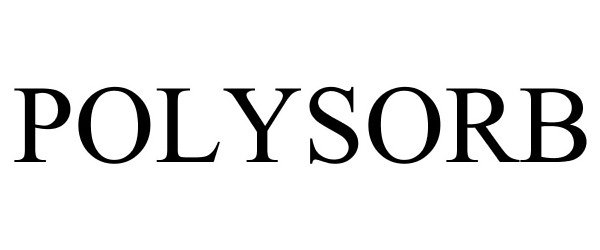 POLYSORB 77860239 3854854 Live/Registered |
IMPLUS FOOTCARE, LLC 2009-10-29 |
 POLYSORB 77754608 3734165 Dead/Cancelled |
IMPLUS FOOTCARE, LLC 2009-06-08 |
 POLYSORB 77008937 3944433 Live/Registered |
Multisorb Technologies, Inc. 2006-09-27 |
 POLYSORB 76116166 not registered Dead/Abandoned |
IMPACT INNOVATIVE PRODUCTS 2000-08-24 |
 POLYSORB 76050784 2560113 Dead/Cancelled |
AquaTex Industries, Inc. 2000-05-17 |
 POLYSORB 75447474 2270475 Live/Registered |
FOUGERA PHARMACEUTICALS INC. 1998-03-10 |
 POLYSORB 75422108 2525414 Dead/Cancelled |
HI-SHEAR CORPORATION 1998-01-23 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.