Sureline 015101
GUDID 10884521640535
SURELINE 02 PEDIATRIC 25UN
ORIDION MEDICAL 1987 LTD.
Carbon-dioxide-sampling nasal oxygen cannula| Primary Device ID | 10884521640535 |
| NIH Device Record Key | 41df49e4-fcca-486e-9c64-ac303f5464f2 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | Sureline |
| Version Model Number | 015101 |
| Catalog Number | 015101 |
| Company DUNS | 600111512 |
| Company Name | ORIDION MEDICAL 1987 LTD. |
| Device Count | 25 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | MR Safe |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | false |
| Serial Number | true |
| Manufacturing Date | true |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
| Phone | +1(508)261-8000 |
| Covidien.UDI@covidien.com | |
| Phone | +1(508)261-8000 |
| Covidien.UDI@covidien.com | |
| Phone | +1(508)261-8000 |
| Covidien.UDI@covidien.com | |
| Phone | +1(508)261-8000 |
| Covidien.UDI@covidien.com | |
| Phone | +1(508)261-8000 |
| Covidien.UDI@covidien.com | |
| Phone | +1(508)261-8000 |
| Covidien.UDI@covidien.com | |
| Phone | +1(508)261-8000 |
| Covidien.UDI@covidien.com | |
| Phone | +1(508)261-8000 |
| Covidien.UDI@covidien.com | |
| Phone | +1(508)261-8000 |
| Covidien.UDI@covidien.com | |
| Phone | +1(508)261-8000 |
| Covidien.UDI@covidien.com | |
| Phone | +1(508)261-8000 |
| Covidien.UDI@covidien.com | |
| Phone | +1(508)261-8000 |
| Covidien.UDI@covidien.com | |
| Phone | +1(508)261-8000 |
| Covidien.UDI@covidien.com | |
| Phone | +1(508)261-8000 |
| Covidien.UDI@covidien.com | |
| Phone | +1(508)261-8000 |
| covidien.udi@covidien.com | |
| Phone | +1(508)261-8000 |
| covidien.udi@covidien.com | |
| Phone | +1(508)261-8000 |
| covidien.udi@covidien.com | |
| Phone | +1(508)261-8000 |
| covidien.udi@covidien.com | |
| Phone | +1(508)261-8000 |
| covidien.udi@covidien.com | |
| Phone | +1(508)261-8000 |
| covidien.udi@covidien.com | |
| Phone | +1(508)261-8000 |
| covidien.udi@covidien.com | |
| Phone | +1(508)261-8000 |
| covidien.udi@covidien.com | |
| Phone | +1(508)261-8000 |
| covidien.udi@covidien.com | |
| Phone | +1(508)261-8000 |
| covidien.udi@covidien.com | |
| Phone | +1(508)261-8000 |
| covidien.udi@covidien.com | |
| Phone | +1(508)261-8000 |
| covidien.udi@covidien.com | |
| Phone | +1(508)261-8000 |
| covidien.udi@covidien.com | |
| Phone | +1(508)261-8000 |
| covidien.udi@covidien.com | |
| Phone | +1(508)261-8000 |
| covidien.udi@covidien.com | |
| Phone | +1(508)261-8000 |
| covidien.udi@covidien.com | |
| Phone | +1(508)261-8000 |
| covidien.udi@covidien.com | |
| Phone | +1(508)261-8000 |
| covidien.udi@covidien.com | |
| Phone | +1(508)261-8000 |
| covidien.udi@covidien.com | |
| Phone | +1(508)261-8000 |
| covidien.udi@covidien.com | |
| Phone | +1(508)261-8000 |
| covidien.udi@covidien.com | |
| Phone | +1(508)261-8000 |
| covidien.udi@covidien.com | |
| Phone | +1(508)261-8000 |
| covidien.udi@covidien.com | |
| Phone | +1(508)261-8000 |
| covidien.udi@covidien.com | |
| Phone | +1(508)261-8000 |
| covidien.udi@covidien.com | |
| Phone | +1(508)261-8000 |
| covidien.udi@covidien.com | |
| Phone | +1(508)261-8000 |
| covidien.udi@covidien.com | |
| Phone | +1(508)261-8000 |
| covidien.udi@covidien.com | |
| Phone | +1(508)261-8000 |
| covidien.udi@covidien.com | |
| Phone | +1(508)261-8000 |
| covidien.udi@covidien.com | |
| Phone | +1(508)261-8000 |
| covidien.udi@covidien.com | |
| Phone | +1(508)261-8000 |
| covidien.udi@covidien.com | |
| Phone | +1(508)261-8000 |
| covidien.udi@covidien.com | |
| Phone | +1(508)261-8000 |
| covidien.udi@covidien.com |
Operating and Storage Conditions
| Special Storage Condition, Specify | Between 0 and 0 *; |
| Special Storage Condition, Specify | Between 0 and 0 *; |
| Special Storage Condition, Specify | Between 0 and 0 *; |
| Special Storage Condition, Specify | Between 0 and 0 *; |
| Special Storage Condition, Specify | Between 0 and 0 *; |
| Special Storage Condition, Specify | Between 0 and 0 *; |
| Special Storage Condition, Specify | Between 0 and 0 *; |
| Special Storage Condition, Specify | Between 0 and 0 *; |
| Special Storage Condition, Specify | Between 0 and 0 *; |
| Special Storage Condition, Specify | Between 0 and 0 *; |
| Special Storage Condition, Specify | Between 0 and 0 *; |
| Special Storage Condition, Specify | Between 0 and 0 *; |
| Special Storage Condition, Specify | Between 0 and 0 *; |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 10884521640535 [Primary] |
| GS1 | 20884521640532 [Unit of Use] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K012395
FDA Product Code
| CCK | Analyzer, Gas, Carbon-Dioxide, Gaseous-Phase |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 6 |
| Public Version Date | 2020-04-01 |
| Device Publish Date | 2016-09-25 |
Devices Manufactured by ORIDION MEDICAL 1987 LTD.
| 10884521741997 - Microstream | 2026-01-09 CAPNOSTREAM 35 PORTABLE RESPIRATORY MONITOR-BRAZIL |
| 10884521520790 - Microstream | 2024-05-06 AC ADAP PKG USA |
| 10884521531512 - Microstream | 2023-12-04 MICROSTREAM ADULT ORAL-NASAL MICROSTREAM FILTERLINE H O2 2M |
| 10884521531536 - Microstream | 2023-12-04 MICROSTREAM ADULT-PED INTUBATED MICROSTREAM FILTERLINE H 2M |
| 10884521531574 - Microstream | 2023-12-04 MICROSTREAM NEONATAL-INFANT INTUBATED MICROSTREAM FILTERLINE |
| 10884521531666 - Microstream | 2023-12-04 MICROSTREAM ADULT ORAL-NASAL MICROSTREAM FILTERLINE 4M 25UN |
| 10884521591073 - Microstream | 2023-09-28 RS09489 |
| 10884521591080 - Microstream | 2023-09-28 RS09603 |
Trademark Results [Sureline]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 SURELINE 87260674 5371423 Live/Registered |
Sunhillo Corporation 2016-12-07 |
 SURELINE 86071606 4581801 Live/Registered |
AGRU/AMERICA, INC. 2013-09-23 |
 SURELINE 77751151 3846218 Live/Registered |
BullsEye Telecom, Inc. 2009-06-03 |
 SURELINE 77485156 not registered Dead/Abandoned |
Promega Corporation 2008-05-28 |
 SURELINE 75870703 2501432 Live/Registered |
DESIGN STANDARDS CORPORATION 1999-12-13 |
 SURELINE 75247697 not registered Dead/Abandoned |
NOMA INC. 1997-02-25 |
 SURELINE 75014982 2025303 Live/Registered |
Apple Bank for Savings 1995-11-03 |
 SURELINE 74356217 not registered Dead/Abandoned |
Seiko Instruments U.S.A., Inc. 1993-02-05 |
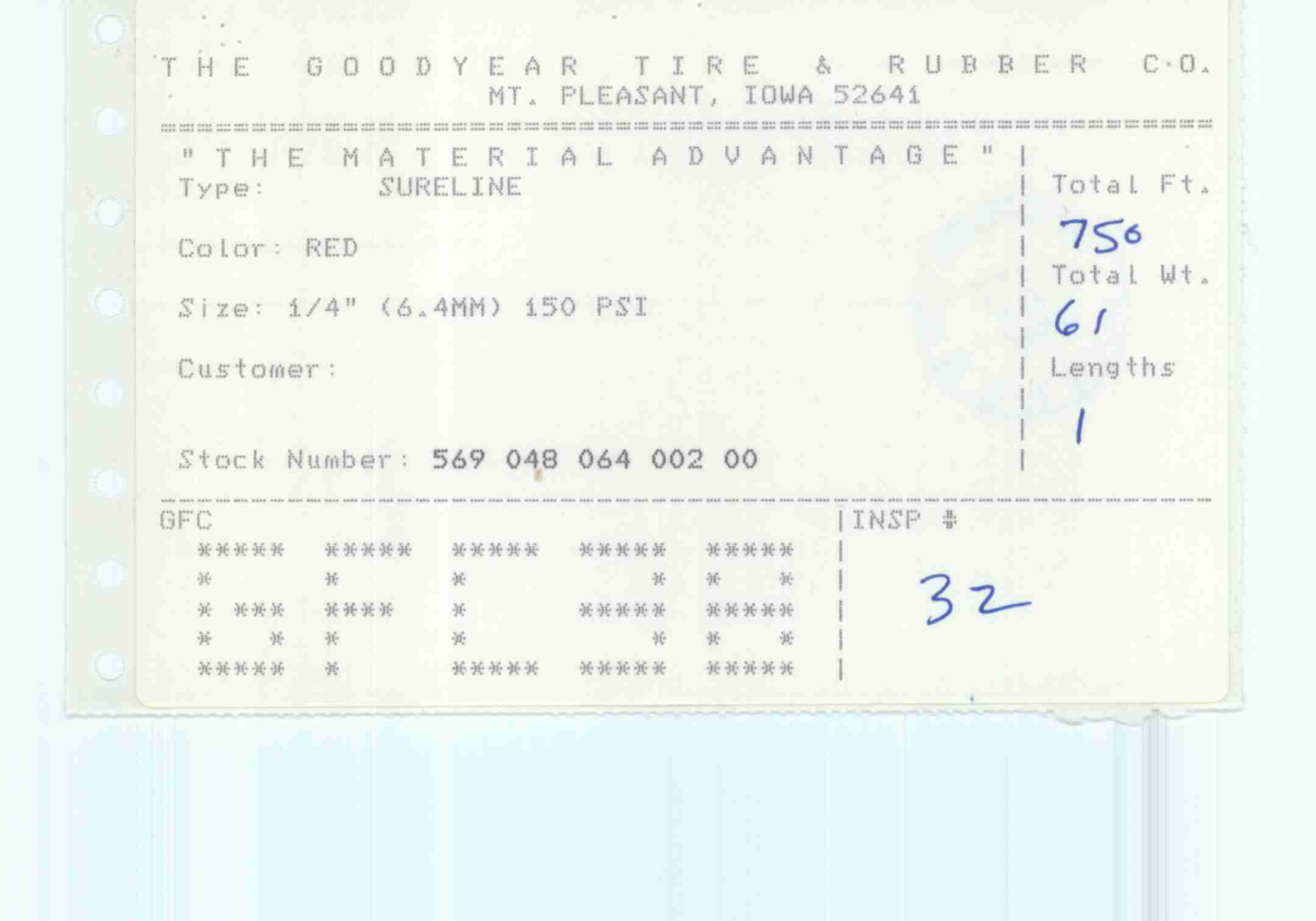 SURELINE 73785697 1565926 Live/Registered |
GOODYEAR TIRE & RUBBER COMPANY, THE 1989-03-10 |
 SURELINE 73492710 1344016 Dead/Cancelled |
IPC COMMUNICATIONS, LTD. 1984-07-31 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.