VAPR 225360
GUDID 10886705009374
VAPR LD Suction Electrode 4 mm x 160 mm
DEPUY MITEK, LLC
Endoscopic electrosurgical electrode, bipolar, single-use| Primary Device ID | 10886705009374 |
| NIH Device Record Key | 231289e6-b525-4cd3-895d-93d0bf277fa0 |
| Commercial Distribution Discontinuation | 2024-05-31 |
| Commercial Distribution Status | Not in Commercial Distribution |
| Brand Name | VAPR |
| Version Model Number | 225360 |
| Catalog Number | 225360 |
| Company DUNS | 190572854 |
| Company Name | DEPUY MITEK, LLC |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
| Phone | +1(800)255-2500 |
| xx@xx.xx | |
| Phone | +1(800)255-2500 |
| xx@xx.xx | |
| Phone | +1(800)255-2500 |
| xx@xx.xx | |
| Phone | +1(800)255-2500 |
| xx@xx.xx | |
| Phone | +1(800)255-2500 |
| xx@xx.xx | |
| Phone | +1(800)255-2500 |
| xx@xx.xx | |
| Phone | +1(800)255-2500 |
| xx@xx.xx | |
| Phone | +1(800)255-2500 |
| xx@xx.xx | |
| Phone | +1(800)255-2500 |
| xx@xx.xx | |
| Phone | +1(800)255-2500 |
| xx@xx.xx | |
| Phone | +1(800)255-2500 |
| xx@xx.xx | |
| Phone | +1(800)255-2500 |
| xx@xx.xx | |
| Phone | +1(800)255-2500 |
| xx@xx.xx | |
| Phone | +1(800)255-2500 |
| xx@xx.xx | |
| Phone | +1(800)255-2500 |
| xx@xx.xx | |
| Phone | +1(800)255-2500 |
| xx@xx.xx | |
| Phone | +1(800)255-2500 |
| xx@xx.xx | |
| Phone | +1(800)255-2500 |
| xx@xx.xx |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 10886705009374 [Primary] |
FDA Product Code
| GEI | ELECTROSURGICAL, CUTTING & COAGULATION & ACCESSORIES |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | true |
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 5 |
| Public Version Date | 2024-06-03 |
| Device Publish Date | 2015-09-01 |
On-Brand Devices [VAPR]
| 10886705009077 | VAPR Sterilization Tray |
| 10886705009107 | VAPR 3 Generator REUSABLE |
| 10886705009381 | VAPR LP Suction Electrode 4 mm x 160 mm |
| 10886705009374 | VAPR LD Suction Electrode 4 mm x 160 mm |
Trademark Results [VAPR]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
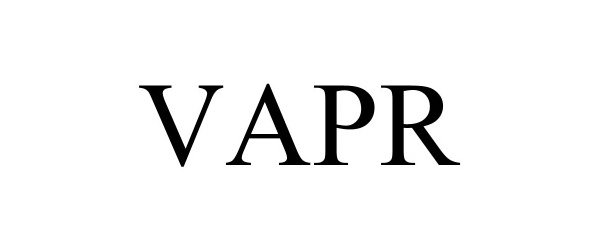 VAPR 98424618 not registered Live/Pending |
MS Sedco, Inc. 2024-02-28 |
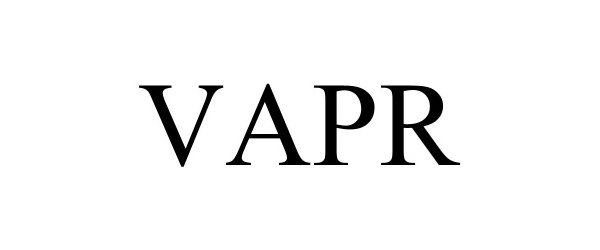 VAPR 98395390 not registered Live/Pending |
Capstan Ag Systems, Inc. 2024-02-07 |
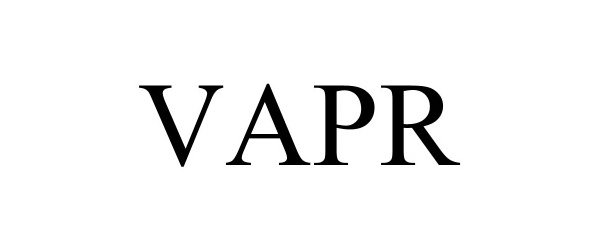 VAPR 97563876 not registered Live/Pending |
Nice Recovery Systems LLC 2022-08-25 |
 VAPR 97066025 not registered Live/Pending |
Industrial Chemicals LLC 2021-10-08 |
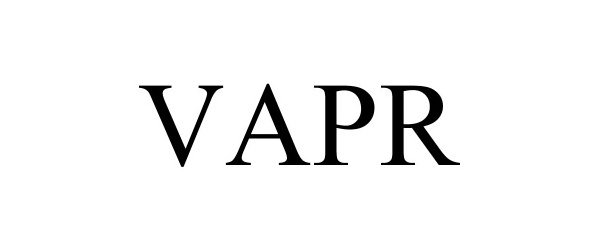 VAPR 97065999 not registered Live/Pending |
Industrial Chemicals LLC 2021-10-08 |
 VAPR 88637942 not registered Live/Pending |
GEODynamics, Inc. 2019-10-01 |
 VAPR 88319705 not registered Live/Pending |
National Oilwell Varco, L.P. 2019-02-28 |
 VAPR 87077624 not registered Dead/Abandoned |
Vaprwear, Gear, LLC 2016-06-20 |
 VAPR 86245969 not registered Dead/Abandoned |
Henderson Aquatics, Inc. 2014-04-08 |
 VAPR 77811937 not registered Dead/Abandoned |
Remington Health Products, LLC 2009-08-25 |
 VAPR 75437271 2295182 Live/Registered |
JOHNSON & JOHNSON 1998-02-19 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.