Balboa™ 19-5525
GUDID 10889981051808
Buttress Screw, 5.5x25mm
SEASPINE ORTHOPEDICS CORPORATION
Spinal fixation plate, non-bioabsorbable| Primary Device ID | 10889981051808 |
| NIH Device Record Key | 130ec091-6b43-4aed-b40d-504f79d08898 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | Balboa™ |
| Version Model Number | 19-5525 |
| Catalog Number | 19-5525 |
| Company DUNS | 079840876 |
| Company Name | SEASPINE ORTHOPEDICS CORPORATION |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
| Phone | +1(760)727-8399 |
| custsvcspine@seaspine.com | |
| Phone | +1(760)727-8399 |
| custsvcspine@seaspine.com | |
| Phone | +1(760)727-8399 |
| custsvcspine@seaspine.com | |
| Phone | +1(760)727-8399 |
| custsvcspine@seaspine.com | |
| Phone | +1(760)727-8399 |
| custsvcspine@seaspine.com | |
| Phone | +1(760)727-8399 |
| custsvcspine@seaspine.com | |
| Phone | +1(760)727-8399 |
| custsvcspine@seaspine.com | |
| Phone | +1(760)727-8399 |
| custsvcspine@seaspine.com | |
| Phone | +1(760)727-8399 |
| custsvcspine@seaspine.com | |
| Phone | +1(760)727-8399 |
| custsvcspine@seaspine.com | |
| Phone | +1(760)727-8399 |
| custsvcspine@seaspine.com | |
| Phone | +1(760)727-8399 |
| custsvcspine@seaspine.com | |
| Phone | +1(760)727-8399 |
| custsvcspine@seaspine.com | |
| Phone | +1(760)727-8399 |
| custsvcspine@seaspine.com | |
| Phone | +1(760)727-8399 |
| custsvcspine@seaspine.com | |
| Phone | +1(760)727-8399 |
| custsvcspine@seaspine.com | |
| Phone | +1(760)727-8399 |
| custsvcspine@seaspine.com | |
| Phone | +1(760)727-8399 |
| custsvcspine@seaspine.com | |
| Phone | +1(760)727-8399 |
| custsvcspine@seaspine.com | |
| Phone | +1(760)727-8399 |
| custsvcspine@seaspine.com | |
| Phone | +1(760)727-8399 |
| custsvcspine@seaspine.com | |
| Phone | +1(760)727-8399 |
| custsvcspine@seaspine.com | |
| Phone | +1(760)727-8399 |
| custsvcspine@seaspine.com | |
| Phone | +1(760)727-8399 |
| custsvcspine@seaspine.com | |
| Phone | +1(760)727-8399 |
| custsvcspine@seaspine.com | |
| Phone | +1(760)727-8399 |
| custsvcspine@seaspine.com | |
| Phone | +1(760)727-8399 |
| custsvcspine@seaspine.com | |
| Phone | +1(760)727-8399 |
| custsvcspine@seaspine.com | |
| Phone | +1(760)727-8399 |
| custsvcspine@seaspine.com |
Device Dimensions
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 10889981051808 [Primary] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K040130
FDA Product Code
| KWQ | APPLIANCE, FIXATION, SPINAL INTERVERTEBRAL BODY |
Sterilization
| Steralize Prior To Use | true |
| Device Is Sterile | false |
[10889981051808]
Moist Heat or Steam Sterilization
[10889981051808]
Moist Heat or Steam Sterilization
[10889981051808]
Moist Heat or Steam Sterilization
[10889981051808]
Moist Heat or Steam Sterilization
[10889981051808]
Moist Heat or Steam Sterilization
[10889981051808]
Moist Heat or Steam Sterilization
[10889981051808]
Moist Heat or Steam Sterilization
[10889981051808]
Moist Heat or Steam Sterilization
[10889981051808]
Moist Heat or Steam Sterilization
[10889981051808]
Moist Heat or Steam Sterilization
[10889981051808]
Moist Heat or Steam Sterilization
[10889981051808]
Moist Heat or Steam Sterilization
[10889981051808]
Moist Heat or Steam Sterilization
[10889981051808]
Moist Heat or Steam Sterilization
[10889981051808]
Moist Heat or Steam Sterilization
[10889981051808]
Moist Heat or Steam Sterilization
[10889981051808]
Moist Heat or Steam Sterilization
[10889981051808]
Moist Heat or Steam Sterilization
[10889981051808]
Moist Heat or Steam Sterilization
[10889981051808]
Moist Heat or Steam Sterilization
[10889981051808]
Moist Heat or Steam Sterilization
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 3 |
| Public Version Date | 2018-07-06 |
| Device Publish Date | 2015-09-24 |
On-Brand Devices [Balboa™]
| 10889981062187 | Balboa Implant |
| 10889981051846 | Buttress Screw 6.5x25mm |
| 10889981051839 | Buttress Screw 6.5x20mm |
| 10889981051822 | Buttress Screw, 5.5x35mm |
| 10889981051815 | Buttress Screw, 5.5x30mm |
| 10889981051808 | Buttress Screw, 5.5x25mm |
| 10889981051792 | Buttress Screw, 5.5x20mm |
| 10889981051785 | Buttress Plate, 15x24mm |
| 10889981051778 | Buttress Plate, 15x19mm |
Trademark Results [Balboa]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
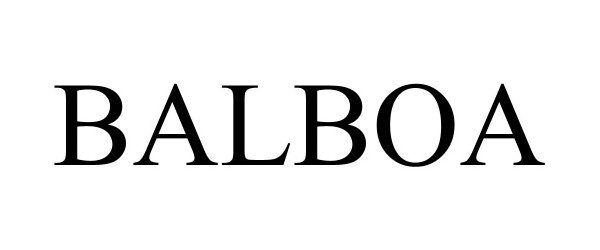 BALBOA 98353495 not registered Live/Pending |
Vanderhall Motor Works, Inc. 2024-01-11 |
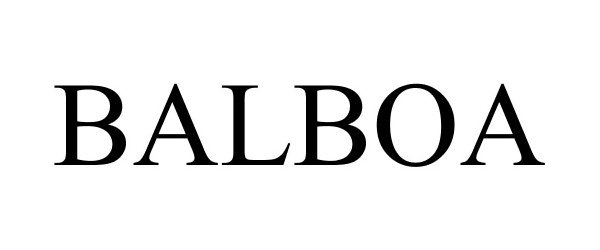 BALBOA 86716957 5074537 Live/Registered |
Arangold Corporation 2015-08-06 |
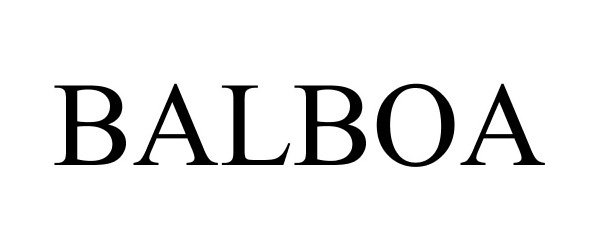 BALBOA 85215495 not registered Dead/Abandoned |
Lamar Branch 2011-01-11 |
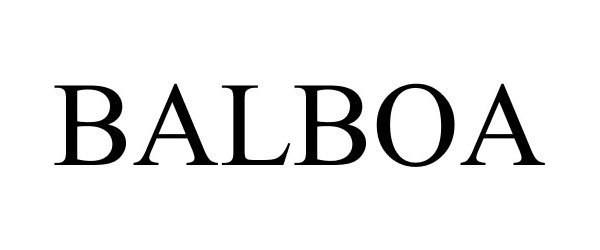 BALBOA 85005568 3902204 Live/Registered |
Hay House, Inc. 2010-04-02 |
 BALBOA 85005565 3971708 Live/Registered |
Hay House, Inc. 2010-04-02 |
 BALBOA 85005561 3971707 Live/Registered |
Hay House, Inc. 2010-04-02 |
 BALBOA 78653816 not registered Dead/Abandoned |
Branch, Lamar 2005-06-20 |
 BALBOA 78618804 3410408 Live/Registered |
BALBOA WINERY, LLC 2005-04-28 |
 BALBOA 77975675 3481992 Dead/Cancelled |
Abercrombie & Fitch Trading Co. 2007-03-29 |
 BALBOA 77828727 3855747 Live/Registered |
Balboa Insurance Company 2009-09-17 |
 BALBOA 77307300 not registered Dead/Abandoned |
Anlin Industries, Inc. 2007-10-18 |
 BALBOA 77280048 3758061 Live/Registered |
BALBOA WATER GROUP, LLC 2007-09-14 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.