MOBI
GUDID 10891040000276
Bluetooth Blood Pressure Monitor
MOBI TECHNOLOGIES, INC.
Automatic-inflation electronic sphygmomanometer, portable, arm/wrist| Primary Device ID | 10891040000276 |
| NIH Device Record Key | bfbfc7d6-5546-4ed1-8702-e5f5e3d2e3a4 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | MOBI |
| Version Model Number | 700027 |
| Company DUNS | 137468570 |
| Company Name | MOBI TECHNOLOGIES, INC. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | false |
| OTC Over-The-Counter | true |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00891040000279 [Primary] |
| GS1 | 10891040000276 [Package] Contains: 00891040000279 Package: Cardboard [6 Units] In Commercial Distribution |
| GS1 | 20891040000273 [Package] Package: Cardboard [24 Units] In Commercial Distribution |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K182495
FDA Product Code
| DXN | System, Measurement, Blood-Pressure, Non-Invasive |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2022-08-08 |
| Device Publish Date | 2022-07-31 |
On-Brand Devices [MOBI]
| 20891040701002 | MOBI Prime3 Ear Thermometer |
| 20891040701675 | MOBI Health Wrist Blood Pressure Monitor (Sphygmomanometers) |
| 20891040702542 | MOBI Health Arm Blood Pressure Monitor (Sphygmomanometers) |
| 20891040701187 | MOBI Air Non-Contact Digital Thermometer |
| 20891040701132 | Non-Contact Thermometer |
| 10891040701111 | Oral Digital Thermometer |
| 10891040702033 | Ultra Pulse Digital Thermometer |
| 20891040701217 | Prime Ear and Forehead Thermometer. |
| 20891040701200 | Ultra Pulse Digital Thermometer |
| 10891040701197 | 70119 |
| 00891040701251 | DualScan Health Check Ear and Forehead Thermometer with Medication Reminder |
| 10891040702996 | MOBI’s Fingertip Pulse Oximeter offers a noninvasive method for monitoring oxygen salutation. |
| 10891040701173 | Air Non-Contact Forehead Thermometer |
| 10891040000306 | Smart Finger Tip Pulse Oximeter |
| 10891040000276 | Bluetooth Blood Pressure Monitor |
| 10891040000269 | Smart DualScan Ear + Forehead Bluetooth Thermometer |
| 10891040701166 | FeverTrack Digital Thermometer |
Trademark Results [MOBI]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
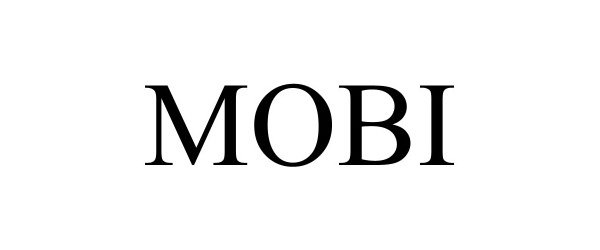 MOBI 98634781 not registered Live/Pending |
Cobionix Corporation 2024-07-05 |
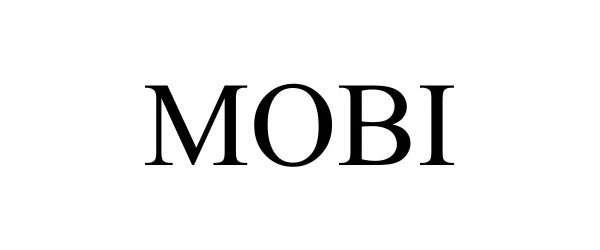 MOBI 98492960 not registered Live/Pending |
Allinox, besloten vennootschap 2024-04-10 |
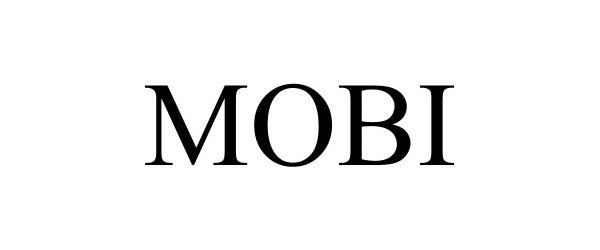 MOBI 98063927 not registered Live/Pending |
Chevron Intellectual Property LLC 2023-06-28 |
 MOBI 90678746 not registered Live/Pending |
Mobi, Inc. 2021-04-28 |
 MOBI 90616340 not registered Live/Pending |
MOBI Antenna Technologies (Shenzhen) Co., Ltd. 2021-03-31 |
 MOBI 90589514 not registered Live/Pending |
Mobi, Inc. 2021-03-19 |
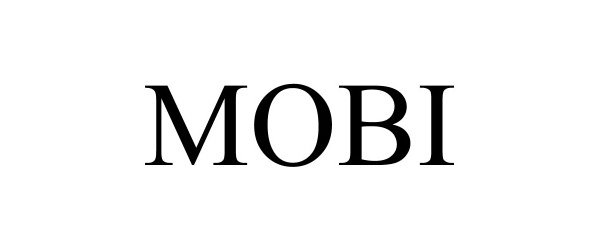 MOBI 90207225 not registered Live/Pending |
Tandem Diabetes Care, Inc. 2020-09-24 |
 MOBI 90090307 not registered Live/Pending |
Realty World, Inc. 2020-08-03 |
 MOBI 90083641 not registered Live/Pending |
PW Branding, Inc. 2020-07-30 |
 MOBI 90083615 not registered Live/Pending |
PW Branding, Inc. 2020-07-30 |
 MOBI 90083590 not registered Live/Pending |
PW Branding, Inc. 2020-07-30 |
 MOBI 90083534 not registered Live/Pending |
PW Branding, Inc. 2020-07-30 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.