Dophi
GUDID 16972304750015
Temperature Probe
Surgnova Healthcare Technologies (Zhejiang) Co., Ltd.
Cryosurgical system temperature probe| Primary Device ID | 16972304750015 |
| NIH Device Record Key | 90833d65-cea6-4cda-9cc1-4ad5a12ac41f |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | Dophi |
| Version Model Number | SS-TP18G-20 |
| Company DUNS | 550274579 |
| Company Name | Surgnova Healthcare Technologies (Zhejiang) Co., Ltd. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | true |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 16972304750015 [Primary] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K183153
FDA Product Code
| NEY | System, Ablation, Microwave And Accessories |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | true |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2022-01-27 |
| Device Publish Date | 2022-01-19 |
On-Brand Devices [Dophi]
| 16972304750282 | Microwave Ablation Electrode Kits |
| 16972304750275 | Microwave Ablation Electrode Kits |
| 16972304750268 | Microwave Ablation Electrode Kits |
| 16972304750251 | Microwave Ablation Electrode Kits |
| 16972304750244 | Microwave Ablation Electrode Kits |
| 16972304750237 | Microwave Ablation Electrode Kits |
| 16972304750015 | Temperature Probe |
| 06972304751435 | Foot Switch |
| 06972304750292 | Microwave Ablaton Generator |
| 16972304750190 | Radio Frequency Electrode Kits |
| 16972304750183 | Radio Frequency Electrode Kits |
| 16972304750176 | Radio Frequency Electrode Kits |
| 16972304750169 | Radio Frequency Electrode Kits |
| 16972304750152 | Radio Frequency Electrode Kits |
| 16972304750145 | Radio Frequency Electrode Kits |
| 16972304750138 | Radio Frequency Electrode Kits |
| 16972304750121 | Radio Frequency Electrode Kits |
| 16972304750114 | Radio Frequency Electrode Kits |
| 16972304750107 | Radio Frequency Electrode Kits |
| 16972304750091 | Radio Frequency Electrode Kits |
| 16972304750084 | Radio Frequency Electrode Kits |
| 16972304750077 | Radio Frequency Electrode Kits |
| 16972304750060 | Radio Frequency Electrode Kits |
| 16972304750053 | Radio Frequency Electrode Kits |
| 16972304750046 | Radio Frequency Electrode Kits |
| 16972304750039 | Radio Frequency Electrode Kits |
| 16972304750022 | Radio Frequency Electrode Kits |
| 06972304751411 | Foot Switch |
| 06972304750209 | Radio Frequency Generator |
Trademark Results [Dophi]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
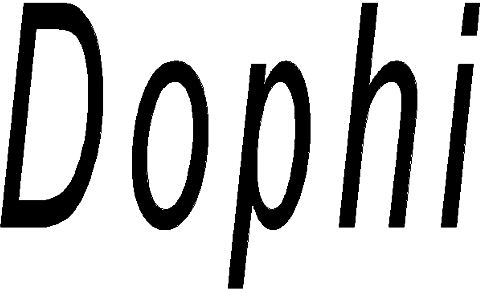 DOPHI 79245790 not registered Live/Pending |
Sinosurgical Healthcare Technologies (Beijing) Co., Ltd. 2018-09-04 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.