EPULSE
GUDID 16978452680020
TENS (Transcutaneous Electric Nerve Stimulation): To be used for temporary relief of pain associated with sore and aching muscles in the shoulder, waist, back,neck,abdomen, bottom, upper extremities (arm), and lower extremities (leg) due to strain from exercise or normal household work activities. EMS (Electrical Muscle Stimulation): It is intended to be used to stimulate healthy muscles in order to improve and facilitate muscle performance.
Shenzhen Jiantuo Electronics Co., Ltd
Physical therapy transcutaneous neuromuscular electrical stimulation system| Primary Device ID | 16978452680020 |
| NIH Device Record Key | 2d00b952-c243-4174-bb97-f4f71f6b8804 |
| Commercial Distribution Discontinuation | 2030-12-02 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | EPULSE |
| Version Model Number | PRO 1200 |
| Company DUNS | 625496494 |
| Company Name | Shenzhen Jiantuo Electronics Co., Ltd |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | false |
| OTC Over-The-Counter | true |
Customer Support Contacts
| Phone | +8618145850692 |
| Sales7@szastec.com | |
| Phone | +8618145850692 |
| Sales7@szastec.com | |
| Phone | +8618145850692 |
| Sales7@szastec.com | |
| Phone | +8618145850692 |
| Sales7@szastec.com | |
| Phone | +8618145850692 |
| Sales7@szastec.com | |
| Phone | +8618145850692 |
| Sales7@szastec.com | |
| Phone | +8618145850692 |
| Sales7@szastec.com | |
| Phone | +8618145850692 |
| Sales7@szastec.com | |
| Phone | +8618145850692 |
| Sales7@szastec.com | |
| Phone | +8618145850692 |
| Sales7@szastec.com | |
| Phone | +8618145850692 |
| Sales7@szastec.com | |
| Phone | +8618145850692 |
| Sales7@szastec.com | |
| Phone | +8618145850692 |
| Sales7@szastec.com |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 06978452680023 [Primary] |
| GS1 | 16978452680020 [Package] Contains: 06978452680023 Package: carton [36 Units] Discontinued: 2025-12-02 Not in Commercial Distribution |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K250360
FDA Product Code
| NUH | Stimulator, Nerve, Transcutaneous, Over-The-Counter |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2025-12-09 |
| Device Publish Date | 2025-12-01 |
Devices Manufactured by Shenzhen Jiantuo Electronics Co., Ltd
| 16978452680020 - EPULSE | 2025-12-09TENS (Transcutaneous Electric Nerve Stimulation): To be used for temporary relief of pain associated with sore and aching muscles in the shoulder, waist, back,neck,abdomen, bottom, upper extremities (arm), and lower extremities (leg) due to strain from exercise or normal household work activities. EMS (Electrical Muscle Stimulation): It is intended to be used to stimulate healthy muscles in order to improve and facilitate muscle performance. |
| 16978452680020 - EPULSE | 2025-12-09 TENS (Transcutaneous Electric Nerve Stimulation): To be used for temporary relief of pain associated with sore and aching muscl |
| 16978452680037 - KONGZEE | 2025-12-09 TENS (Transcutaneous Electric Nerve Stimulation): To be used for temporary relief of pain associated with sore and aching muscl |
| 16978452680013 - NURSAL | 2025-08-29 TENS (Transcutaneous Electric Nerve Stimulation): To be used for temporary relief of pain associated with sore and aching muscl |
Trademark Results [EPULSE]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 EPULSE 97097344 not registered Live/Pending |
Epulse LLC 2021-10-28 |
 EPULSE 86529986 not registered Dead/Abandoned |
Pulse iQ, Inc. 2015-02-10 |
 EPULSE 86515091 4865688 Live/Registered |
ePulse Limited 2015-01-27 |
 EPULSE 86097681 4676375 Live/Registered |
Mueller International, LLC 2013-10-22 |
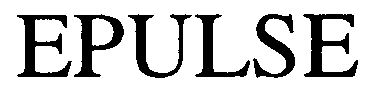 EPULSE 79018481 3209368 Dead/Cancelled |
ePULSE Limited 2004-10-12 |
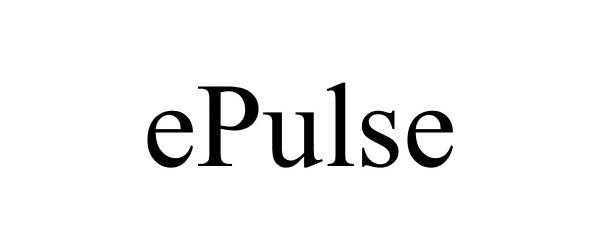 EPULSE 77747722 not registered Dead/Abandoned |
Enalasys, Inc. 2009-05-29 |
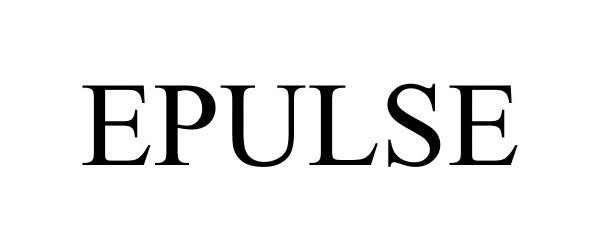 EPULSE 77228085 3385180 Live/Registered |
Primordial Diagnostics, Inc. 2007-07-12 |
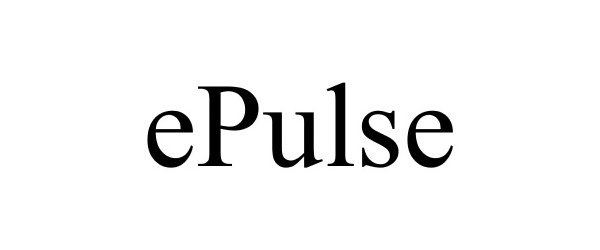 EPULSE 77002672 3313246 Dead/Cancelled |
Impact Sports Technologies Inc. 2006-09-19 |
 EPULSE 76052070 not registered Dead/Abandoned |
InterX Technologies. Inc. 2000-05-19 |
 EPULSE 75271138 2149377 Dead/Cancelled |
MTS, Incorporated 1997-04-07 |
 EPULSE 74707808 1993203 Dead/Cancelled |
MTS, Incorporated 1995-07-28 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.