InSpace™ 0131
GUDID 17290013396058
InSpace™ system, Medium The InSpace™ Subacromial Tissue Spacer System is a Shoulder spacer for massive irreparable rotator cuff tears, resorbable, inflatable, non-fixed. The InSpace™ Subacromial Tissue Spacer System implant is designed to act as a spacer, creating a physical barrier between tissues in the subacromial space. It is supplied sterile (EtO) and intended for single use.
ORTHO-SPACE LTD
Tendon spacer| Primary Device ID | 17290013396058 |
| NIH Device Record Key | 710d4597-8b48-4531-85ab-eb4886687a01 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | InSpace™ |
| Version Model Number | 0131 |
| Catalog Number | 0131 |
| Company DUNS | 533152083 |
| Company Name | ORTHO-SPACE LTD |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | MR Safe |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Operating and Storage Conditions
| Storage Environment Temperature | Between 0 Degrees Celsius and 29 Degrees Celsius |
| Storage Environment Temperature | Between 0 Degrees Celsius and 29 Degrees Celsius |
| Storage Environment Temperature | Between 0 Degrees Celsius and 29 Degrees Celsius |
| Storage Environment Temperature | Between 0 Degrees Celsius and 29 Degrees Celsius |
| Storage Environment Temperature | Between 0 Degrees Celsius and 29 Degrees Celsius |
| Storage Environment Temperature | Between 0 Degrees Celsius and 29 Degrees Celsius |
| Storage Environment Temperature | Between 0 Degrees Celsius and 29 Degrees Celsius |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 17290013396058 [Primary] |
FDA Pre-market Approvals/Notifications & deNovo
- deNovo: DEN200039
FDA Product Code
| QPQ | Shoulder Spacer For Massive Irreparable Rotator Cuff Tear, Resorbable, Inflatable, Non-Fixed |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | true |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2021-08-09 |
| Device Publish Date | 2021-08-01 |
On-Brand Devices [InSpace™]
| 17290013396065 | InSpace™ system, Large The InSpace™ Subacromial Tissue Spacer System is a Shoulder spacer fo |
| 17290013396058 | InSpace™ system, Medium The InSpace™ Subacromial Tissue Spacer System is a Shoulder spacer f |
| 17290013396041 | InSpace™ system Small The InSpace™ Subacromial Tissue Spacer System is a Shoulder spacer for |
Trademark Results [InSpace]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
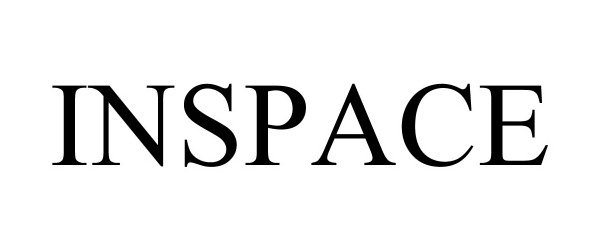 INSPACE 97171681 not registered Live/Pending |
Inspace, Inc. 2021-12-14 |
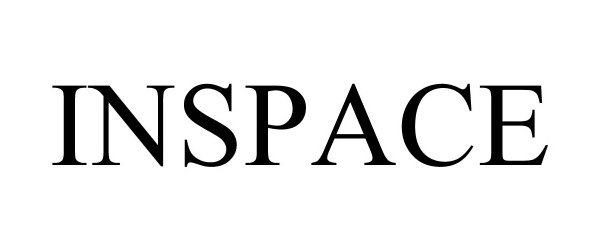 INSPACE 90842184 not registered Live/Pending |
Inspace, Inc. 2021-07-22 |
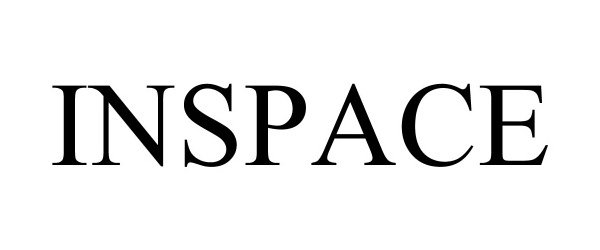 INSPACE 90689417 not registered Live/Pending |
InSpace Proximity, Inc. 2021-05-04 |
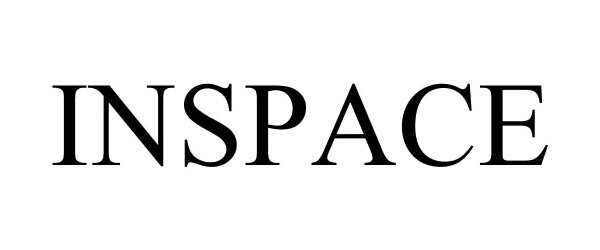 INSPACE 86275958 4856367 Live/Registered |
Ortho-Space Ltd 2014-05-08 |
 INSPACE 85082988 not registered Dead/Abandoned |
Ortho-Space Ltd 2010-07-13 |
 INSPACE 76110305 2657909 Dead/Cancelled |
Infinite Space Systems, Inc. 2000-08-16 |
 INSPACE 73309989 1206485 Dead/Cancelled |
International Space Risk Management Systems 1981-05-12 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.