MOVES
GUDID 18071411006959
Thornhill Research Inc
Transport electric ventilator| Primary Device ID | 18071411006959 |
| NIH Device Record Key | 865d1e02-c7a7-4f09-8253-ed270b7f23ae |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | MOVES |
| Version Model Number | 100695 |
| Company DUNS | 205151975 |
| Company Name | Thornhill Research Inc |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | true |
| Manufacturing Date | true |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
| Phone | 888.597.1325 |
| info@thornhillresearch.com | |
| Phone | 888.597.1325 |
| info@thornhillresearch.com |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 18071411006959 [Primary] |
| GS1 | 18071411243514 [Package] Package: Box [6 Units] In Commercial Distribution |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K093261
FDA Product Code
| BTL | Ventilator, Emergency, Powered (Resuscitator) |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 3 |
| Public Version Date | 2018-07-06 |
| Device Publish Date | 2015-11-06 |
On-Brand Devices [MOVES]
| 18071411242418 | Hose, 0.04" x 0.0625", Nafion w/ Label |
| 18071411008168 | 100816 |
| 18071411115590 | 111559 |
| 18071411114227 | 111422 |
| 18071411243538 | 101211 |
| 18071411243521 | 101210 |
| 18071411008236 | 100823 |
| 18071411243507 | 100696 |
| 18071411006959 | 100695 |
| 18071411006270 | 100627 |
| 18071411006263 | 100626 |
| 10807141000169 | MOVES Clamps |
| 10807141000152 | MOVES Clamps |
| 10807141000145 | Assy, Power Supply Charger Unit |
| 10807141000138 | Assy, Battery Pack |
| 10807141000084 | Hose, 0.04" x 0.0625", Nafion w/ Label |
| 10807141000077 | ASSY, MOVES System Case |
| 10807141000060 | ASSY, MOVES Accessory Case |
| 20807141000050 | Assy, O2 Mask Breathing Circuit |
| 20807141000036 | Assy, Ventilator Breathing Circuit |
| 10807141000015 | Assy, O2 Mask Cartridge |
| 10807141000008 | Assy, Ventilator Cartridge |
Trademark Results [MOVES]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 MOVES 98166389 not registered Live/Pending |
Rothbury Wines Pty Ltd 2023-09-06 |
 MOVES 97575955 not registered Live/Pending |
MOVEZ TECHNOLOGIES, INC. 2022-09-01 |
 MOVES 97341594 not registered Live/Pending |
Madeline Moves, Inc. 2022-03-31 |
 MOVES 97151470 not registered Live/Pending |
Assembled Financial Technology LLC 2021-12-01 |
 MOVES 88925391 not registered Live/Pending |
Moves Managed Services Inc. 2020-05-20 |
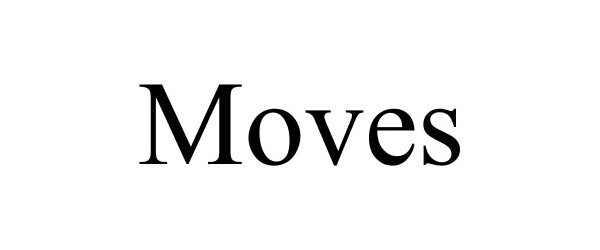 MOVES 88384588 not registered Live/Pending |
Coleman, John C 2019-04-13 |
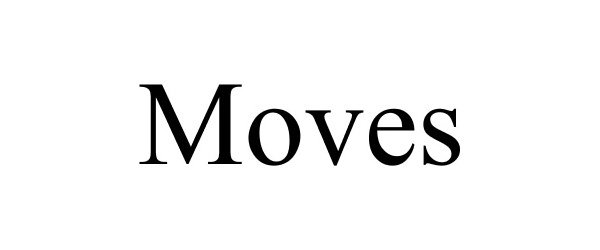 MOVES 87849067 5909092 Live/Registered |
Movez Technologies, Inc. 2018-03-26 |
 MOVES 87365134 5261291 Live/Registered |
Coty Brands Management Inc. 2017-03-09 |
 MOVES 79293990 not registered Live/Pending |
Boschung Mecatronic SA 2020-06-29 |
 MOVES 79271107 not registered Live/Pending |
MVS IN MOTION BVBA 2018-12-05 |
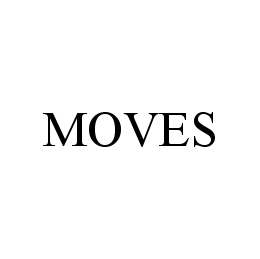 MOVES 78421579 3001352 Live/Registered |
MOVES DANCE STUDIO, INC. 2004-05-19 |
 MOVES 78311578 not registered Dead/Abandoned |
Moves Media Group, LLC 2003-10-09 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.