Collage
GUDID 18257200121878
Osteoconductive Scaffold Strip, 15cc
ORTHOFIX INC.
Bone matrix implant, composite| Primary Device ID | 18257200121878 |
| NIH Device Record Key | c6bc4a79-1dc2-4c57-83df-20b7cda46c71 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | Collage |
| Version Model Number | 711015 |
| Company DUNS | 927083808 |
| Company Name | ORTHOFIX INC. |
| Device Count | 1 |
| DM Exempt | true |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | true |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | true |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
Operating and Storage Conditions
| Storage Environment Temperature | Between 50 Degrees Fahrenheit and 77 Degrees Fahrenheit |
| Storage Environment Temperature | Between 50 Degrees Fahrenheit and 77 Degrees Fahrenheit |
| Storage Environment Temperature | Between 50 Degrees Fahrenheit and 77 Degrees Fahrenheit |
| Storage Environment Temperature | Between 50 Degrees Fahrenheit and 77 Degrees Fahrenheit |
| Storage Environment Temperature | Between 50 Degrees Fahrenheit and 77 Degrees Fahrenheit |
| Storage Environment Temperature | Between 50 Degrees Fahrenheit and 77 Degrees Fahrenheit |
| Storage Environment Temperature | Between 50 Degrees Fahrenheit and 77 Degrees Fahrenheit |
| Storage Environment Temperature | Between 50 Degrees Fahrenheit and 77 Degrees Fahrenheit |
| Storage Environment Temperature | Between 50 Degrees Fahrenheit and 77 Degrees Fahrenheit |
| Storage Environment Temperature | Between 50 Degrees Fahrenheit and 77 Degrees Fahrenheit |
| Storage Environment Temperature | Between 50 Degrees Fahrenheit and 77 Degrees Fahrenheit |
| Storage Environment Temperature | Between 50 Degrees Fahrenheit and 77 Degrees Fahrenheit |
| Storage Environment Temperature | Between 50 Degrees Fahrenheit and 77 Degrees Fahrenheit |
| Storage Environment Temperature | Between 50 Degrees Fahrenheit and 77 Degrees Fahrenheit |
| Storage Environment Temperature | Between 50 Degrees Fahrenheit and 77 Degrees Fahrenheit |
| Storage Environment Temperature | Between 50 Degrees Fahrenheit and 77 Degrees Fahrenheit |
| Storage Environment Temperature | Between 50 Degrees Fahrenheit and 77 Degrees Fahrenheit |
| Storage Environment Temperature | Between 50 Degrees Fahrenheit and 77 Degrees Fahrenheit |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 18257200121878 [Primary] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K063124
FDA Product Code
| MQV | Filler, Bone Void, Calcium Compound |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | true |
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 3 |
| Public Version Date | 2018-07-06 |
| Device Publish Date | 2016-09-14 |
On-Brand Devices [Collage ]
| 18257200121878 | Osteoconductive Scaffold Strip, 15cc |
| 18257200121861 | Osteoconductive Scaffold Strip 10 cc |
| 18257200115136 | Collage Osteoconductive Scaffold Putty 15cc |
| 18257200115129 | Collage Osteoconductive Scaffold Putty 10 cc |
| 18257200115112 | Collage Osteoconductive Scaffold Putty, 5cc |
Trademark Results [Collage]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 COLLAGE 98824432 not registered Live/Pending |
Collage Bio Inc. 2024-10-28 |
 COLLAGE 98623353 not registered Live/Pending |
J & R SOUTH COAST PLAZA, LLC 2024-06-27 |
 COLLAGE 98487297 not registered Live/Pending |
Collage Labs Inc 2024-04-06 |
 COLLAGE 98472732 not registered Live/Pending |
Aperture Cellars, LLC 2024-03-28 |
 COLLAGE 98071531 not registered Live/Pending |
Kohler Co. 2023-07-05 |
 COLLAGE 97056114 not registered Live/Pending |
Aquilini Brands USA Inc. 2021-10-01 |
 COLLAGE 90568398 not registered Live/Pending |
Clear Touch Interactive, Inc. 2021-03-09 |
 COLLAGE 90380269 not registered Live/Pending |
Project Miracle IP Holdings, LLC 2020-12-14 |
 COLLAGE 90042056 not registered Live/Pending |
J & R South Coast Plaza, LLC 2020-07-08 |
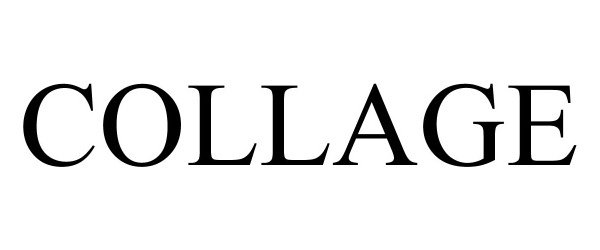 COLLAGE 88702112 not registered Live/Pending |
Stathy, Inc. 2019-11-21 |
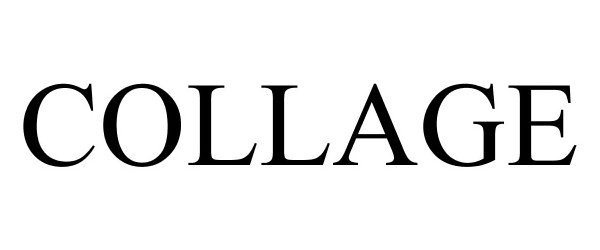 COLLAGE 88159856 not registered Live/Pending |
Collage LLC 2018-10-18 |
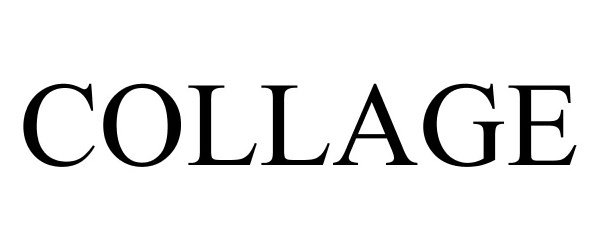 COLLAGE 87398851 not registered Dead/Abandoned |
Hagafen Cellars 2017-04-05 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.