AGNES
GUDID 18800067700009
This is 1 set with following device AGNES: 1EA, GA-B: 10EA, GA-C: 10EA, GA-E: 10EA, GA-F1A: 10EA, GA-F1B: 10EA, GA-I: 10EA, GA-IL: 10EA, GA-S: 10EA, GA-SL: 10EA GA-W3A: 10EA, GA-F3A: 10EA, GA-W3B: 10EA AG-CN18G: 10EA, AG-CN19G: 10EA, AG-CN20G: 10EA, AG-CN21G: 10EA, AG-CN23G: 10EA
AGNES MEDICAL CO., LTD.
Intradermal radio-frequency ablation system| Primary Device ID | 18800067700009 |
| NIH Device Record Key | e2e7cce7-f5f3-4356-bd2c-d9e1ac4c5d25 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | AGNES |
| Version Model Number | AGNES |
| Company DUNS | 695070346 |
| Company Name | AGNES MEDICAL CO., LTD. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | false |
| Serial Number | true |
| Manufacturing Date | true |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
| Phone | +823180209717 |
| jglee@agnesmedical.com | |
| Phone | +823180209717 |
| jglee@agnesmedical.com | |
| Phone | +823180209717 |
| jglee@agnesmedical.com | |
| Phone | +823180209717 |
| jglee@agnesmedical.com | |
| Phone | +823180209717 |
| jglee@agnesmedical.com | |
| Phone | +823180209717 |
| jglee@agnesmedical.com | |
| Phone | +823180209717 |
| jglee@agnesmedical.com | |
| Phone | +823180209717 |
| jglee@agnesmedical.com | |
| Phone | +823180209717 |
| jglee@agnesmedical.com | |
| Phone | +823180209717 |
| jglee@agnesmedical.com | |
| Phone | +823180209717 |
| jglee@agnesmedical.com | |
| Phone | +823180209717 |
| jglee@agnesmedical.com | |
| Phone | +823180209717 |
| jglee@agnesmedical.com | |
| Phone | +823180209717 |
| jglee@agnesmedical.com | |
| Phone | +823180209717 |
| jglee@agnesmedical.com | |
| Phone | +823180209717 |
| jglee@agnesmedical.com | |
| Phone | +823180209717 |
| jglee@agnesmedical.com |
Operating and Storage Conditions
| Storage Environment Temperature | Between 0 Degrees Celsius and 40 Degrees Celsius |
| Storage Environment Humidity | Between 0 Percent (%) Relative Humidity and 80 Percent (%) Relative Humidity |
| Storage Environment Temperature | Between 0 Degrees Celsius and 40 Degrees Celsius |
| Storage Environment Humidity | Between 0 Percent (%) Relative Humidity and 80 Percent (%) Relative Humidity |
| Storage Environment Temperature | Between 0 Degrees Celsius and 40 Degrees Celsius |
| Storage Environment Humidity | Between 0 Percent (%) Relative Humidity and 80 Percent (%) Relative Humidity |
| Storage Environment Temperature | Between 0 Degrees Celsius and 40 Degrees Celsius |
| Storage Environment Humidity | Between 0 Percent (%) Relative Humidity and 80 Percent (%) Relative Humidity |
| Storage Environment Temperature | Between 0 Degrees Celsius and 40 Degrees Celsius |
| Storage Environment Humidity | Between 0 Percent (%) Relative Humidity and 80 Percent (%) Relative Humidity |
| Storage Environment Temperature | Between 0 Degrees Celsius and 40 Degrees Celsius |
| Storage Environment Humidity | Between 0 Percent (%) Relative Humidity and 80 Percent (%) Relative Humidity |
| Storage Environment Temperature | Between 0 Degrees Celsius and 40 Degrees Celsius |
| Storage Environment Humidity | Between 0 Percent (%) Relative Humidity and 80 Percent (%) Relative Humidity |
| Storage Environment Temperature | Between 0 Degrees Celsius and 40 Degrees Celsius |
| Storage Environment Humidity | Between 0 Percent (%) Relative Humidity and 80 Percent (%) Relative Humidity |
| Storage Environment Temperature | Between 0 Degrees Celsius and 40 Degrees Celsius |
| Storage Environment Humidity | Between 0 Percent (%) Relative Humidity and 80 Percent (%) Relative Humidity |
| Storage Environment Temperature | Between 0 Degrees Celsius and 40 Degrees Celsius |
| Storage Environment Humidity | Between 0 Percent (%) Relative Humidity and 80 Percent (%) Relative Humidity |
| Storage Environment Temperature | Between 0 Degrees Celsius and 40 Degrees Celsius |
| Storage Environment Humidity | Between 0 Percent (%) Relative Humidity and 80 Percent (%) Relative Humidity |
| Storage Environment Temperature | Between 0 Degrees Celsius and 40 Degrees Celsius |
| Storage Environment Humidity | Between 0 Percent (%) Relative Humidity and 80 Percent (%) Relative Humidity |
| Storage Environment Temperature | Between 0 Degrees Celsius and 40 Degrees Celsius |
| Storage Environment Humidity | Between 0 Percent (%) Relative Humidity and 80 Percent (%) Relative Humidity |
| Storage Environment Temperature | Between 0 Degrees Celsius and 40 Degrees Celsius |
| Storage Environment Humidity | Between 0 Percent (%) Relative Humidity and 80 Percent (%) Relative Humidity |
| Storage Environment Temperature | Between 0 Degrees Celsius and 40 Degrees Celsius |
| Storage Environment Humidity | Between 0 Percent (%) Relative Humidity and 80 Percent (%) Relative Humidity |
| Storage Environment Temperature | Between 0 Degrees Celsius and 40 Degrees Celsius |
| Storage Environment Humidity | Between 0 Percent (%) Relative Humidity and 80 Percent (%) Relative Humidity |
| Storage Environment Temperature | Between 0 Degrees Celsius and 40 Degrees Celsius |
| Storage Environment Humidity | Between 0 Percent (%) Relative Humidity and 80 Percent (%) Relative Humidity |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 18800067700009 [Primary] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K203013
FDA Product Code
| GEI | Electrosurgical, Cutting & Coagulation & Accessories |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2023-07-24 |
| Device Publish Date | 2023-07-14 |
On-Brand Devices [AGNES]
| 18800067700009 | This is 1 set with following device AGNES: 1EA, GA-B: 10EA, GA-C: 10EA, GA-E: 10EA, GA-F1A: 10EA |
| 08800067700002 | AGNES |
Trademark Results [AGNES]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
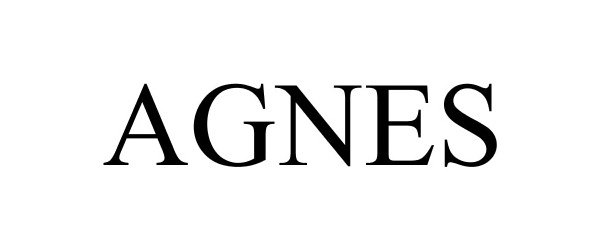 AGNES 88476473 5949738 Live/Registered |
Lindsey Adelman Studio LLC 2019-06-17 |
 AGNES 88346432 5895046 Live/Registered |
AMD Global Telemedicine, Inc. 2019-03-19 |
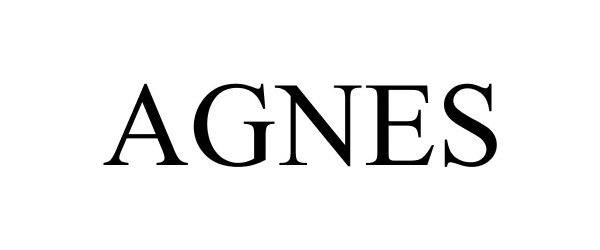 AGNES 86834011 5080576 Live/Registered |
AGNES MEDICAL CO., LTD. 2015-11-30 |
 AGNES 86069496 not registered Dead/Abandoned |
Del Boca, Adrian, M.D., P.A. 2013-09-19 |
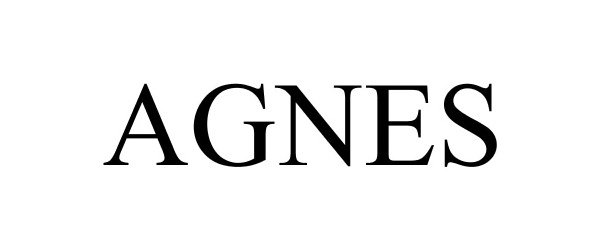 AGNES 85957733 4509648 Live/Registered |
AMD Global Telemedicine 2013-06-12 |
 AGNES 77833633 not registered Dead/Abandoned |
Del Boca, Adrian, M.D., P.A. 2009-09-23 |
 AGNES 73454430 1301909 Dead/Cancelled |
CBS Inc. 1983-11-25 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.