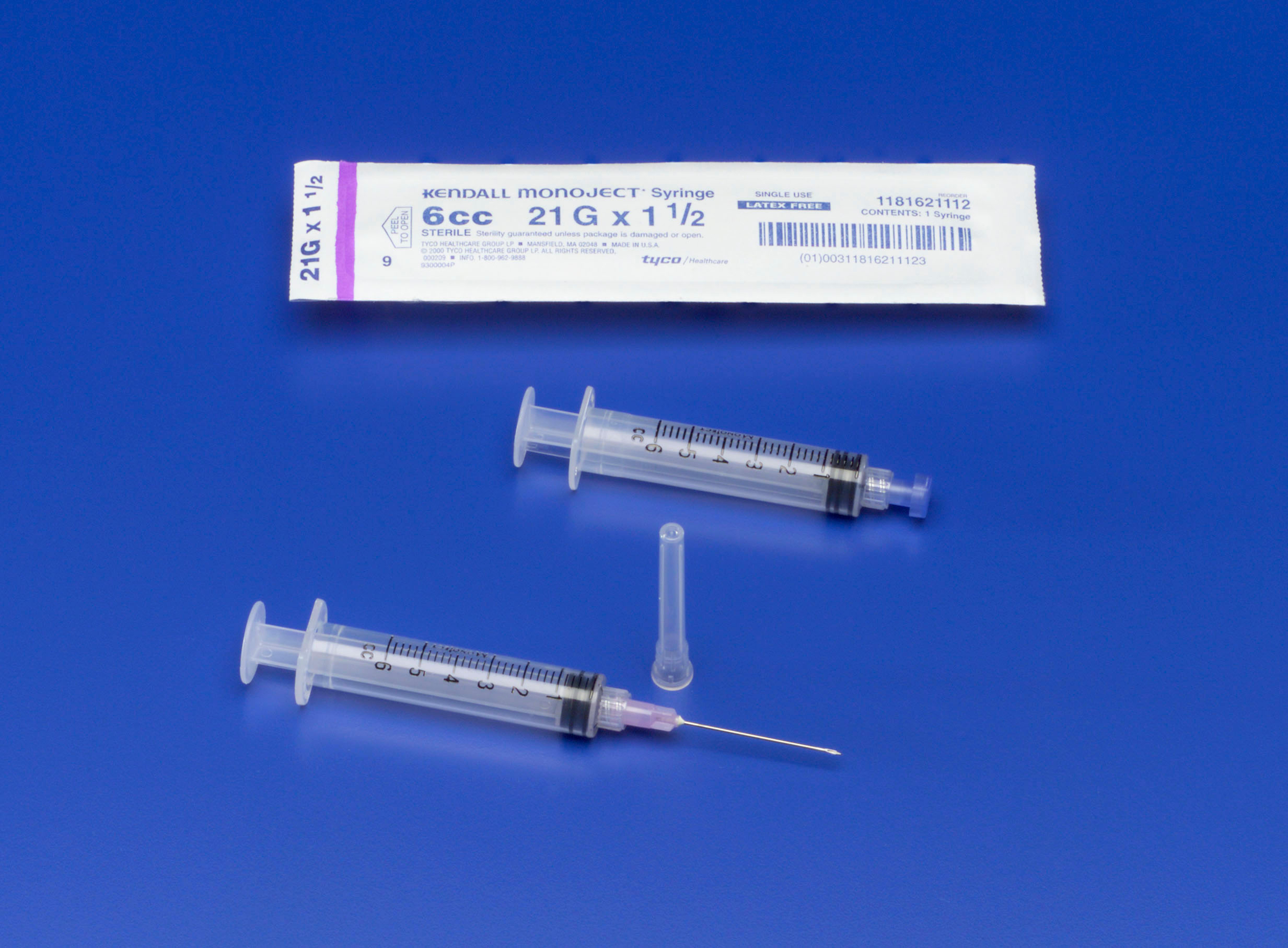
Monoject 1181200777T
GUDID 20192253025818
12 mL Syringe Luer-Lock Tip
Cardinal Health, Inc.
General-purpose syringe, single-use| Primary Device ID | 20192253025818 |
| NIH Device Record Key | 20f4284f-fe7a-4ad5-af9e-25e16e7fc43e |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | Monoject |
| Version Model Number | 1181200777T |
| Catalog Number | 1181200777T |
| Company DUNS | 080935429 |
| Company Name | Cardinal Health, Inc. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 10192253025811 [Primary] |
| GS1 | 20192253025818 [Package] Contains: 10192253025811 Package: Carton [100 Units] In Commercial Distribution |
| GS1 | 50192253025819 [Package] Package: CASE [10 Units] In Commercial Distribution |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K113091
FDA Product Code
| FMF | Syringe, piston |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | true |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2022-11-07 |
| Device Publish Date | 2022-10-28 |
On-Brand Devices [Monoject]
| 30884527022742 | Insulin Syringe |
| 30884527022735 | Insulin Syringe |
| 20884527022721 | Insulin Syringe |
| 20884527022714 | Insulin Syringe |
| 00884521169541 | Insulin Syringe |
| 00884521169527 | Insulin Syringe |
| 00884521169503 | Insulin Syringe |
| 20884527022677 | Insulin Syringe |
| 30884527022667 | Insulin Syringe |
| 00884521169442 | Insulin Syringe |
| 10884527022649 | Insulin Syringe |
| 20884521151847 | Insulin Syringe with Extra Fine Needle |
| 20884521151830 | Insulin Syringe with Extra Fine Needle |
| 20884521151823 | Insulin Syringe with Extra Fine Needle |
| 30884521010431 | Endodontic Needle |
| 20884521005690 | 0.9% Sodium Chloride,Flush Syringe, 2.5 mL Fill, Blunt Cannula |
| 10884527017966 | Filter Needle,Aluminum Hub |
| 20884527006752 | Blood Collection Tube,Silicone Coated Blue Stopper, Buffered Sodium Citrate 0.5 mL 3.2% Solution |
| 10884527006632 | Blood Collection Tube,Silicone Coated Lavender Stopper, EDTA (K3) 0.05 mL 15.0% Solution, 5 mL D |
| 10884521163706 | 0.9% Sodium Chloride,Flush Syringe, 2.5 mL Fill |
| 10884521163690 | 0.9% Sodium Chloride,Flush Syringe, 5 mL Fill |
| 10884521163683 | 0.9% Sodium Chloride,Flush Syringe, 3 mL Fill |
| 10884521163676 | 0.9% Sodium Chloride,Flush Syringe, 10 mL Fill |
| 20884521150093 | Blood Collection Tube,Glycerine Coated Lavender Stopper, EDTA (K3) 0.04 mL 15.0% Solution, 4.0 m |
| 20884521102757 | Needleless Med Prep Cannula |
| 20884521102498 | Enteral Syringe with Tip Cap |
| 20884521082929 | Blunt Tip Cannula,Vial Access Pin |
| 20884521082493 | Vial Access Cannula,Smart Tip |
| 10884521082472 | Vial Access Combo,Blunt Tip |
| 10884521015050 | 1 mL Insulin Syringe,Permanent Needle |
| 20884521015033 | 1 mL Insulin Syringe,Permanent Needle |
| 10884521015029 | 1 mL Insulin Syringe,Permanent Needle |
| 10884521015005 | 3/10 mL Insulin Syringe,Permanent Needle |
| 10884521014985 | 1/2 mL Insulin Syringe,Permanent Needle |
| 10884521014923 | 1/2 mL Insulin Syringe,Permanent Needle |
| 10884521014909 | 1/2 mL Insulin Syringe,Permanent Needle |
| 10884521014480 | Safety Syringe Tip Cap |
| 20884521013909 | 1/2 mL Tuberculin Syringe,Permanent Needle |
| 20884521013077 | Blood Collection/Infusion Set,Female Luer, 12 inch (30.5 cm) Tubing |
| 20884521012995 | Blood Collection/Infusion Set,Female Luer, 12 inch (30.5 cm) Tubing |
| 20884521012971 | Blood Collection/Infusion Set,Female Luer, 12 inch (30.5 cm) Tubing |
| 20884521008790 | Standard Hypodermic Needle,Aluminum Hub |
| 20884521008752 | Standard Hypodermic Needle,Aluminum Hub |
| 20884521008745 | Standard Hypodermic Needle,Aluminum Hub |
| 20884521008707 | Standard Hypodermic Needle,Aluminum Hub |
| 20884521008646 | Standard Hypodermic Needle,Aluminum Hub |
| 20884521008585 | Standard Hypodermic Needle,Aluminum Hub |
| 20884521008547 | Standard Hypodermic Needle,Aluminum Hub |
| 20884521008431 | Standard Hypodermic Needle,Aluminum Hub |
| 20884521008387 | Standard Hypodermic Needle,Aluminum Hub |
Trademark Results [Monoject]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 MONOJECT 74560228 2099750 Dead/Cancelled |
SHERWOOD SERVICES AG 1994-08-08 |
 MONOJECT 74191238 1762511 Dead/Cancelled |
Ecolab Inc. 1991-08-05 |
 MONOJECT 73569258 1403009 Live/Registered |
SHERWOOD MEDICAL COMPANY 1985-11-18 |
 MONOJECT 73569236 1403008 Live/Registered |
SHERWOOD MEDICAL COMPANY 1985-11-18 |
 MONOJECT 73170520 1124445 Dead/Cancelled |
SHERWOOD MEDICAL INDUSTRIES INC. 1978-05-15 |
 MONOJECT 72037194 0664129 Dead/Expired |
ROEHR PRODUCTS CO., INC. 1957-09-13 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.