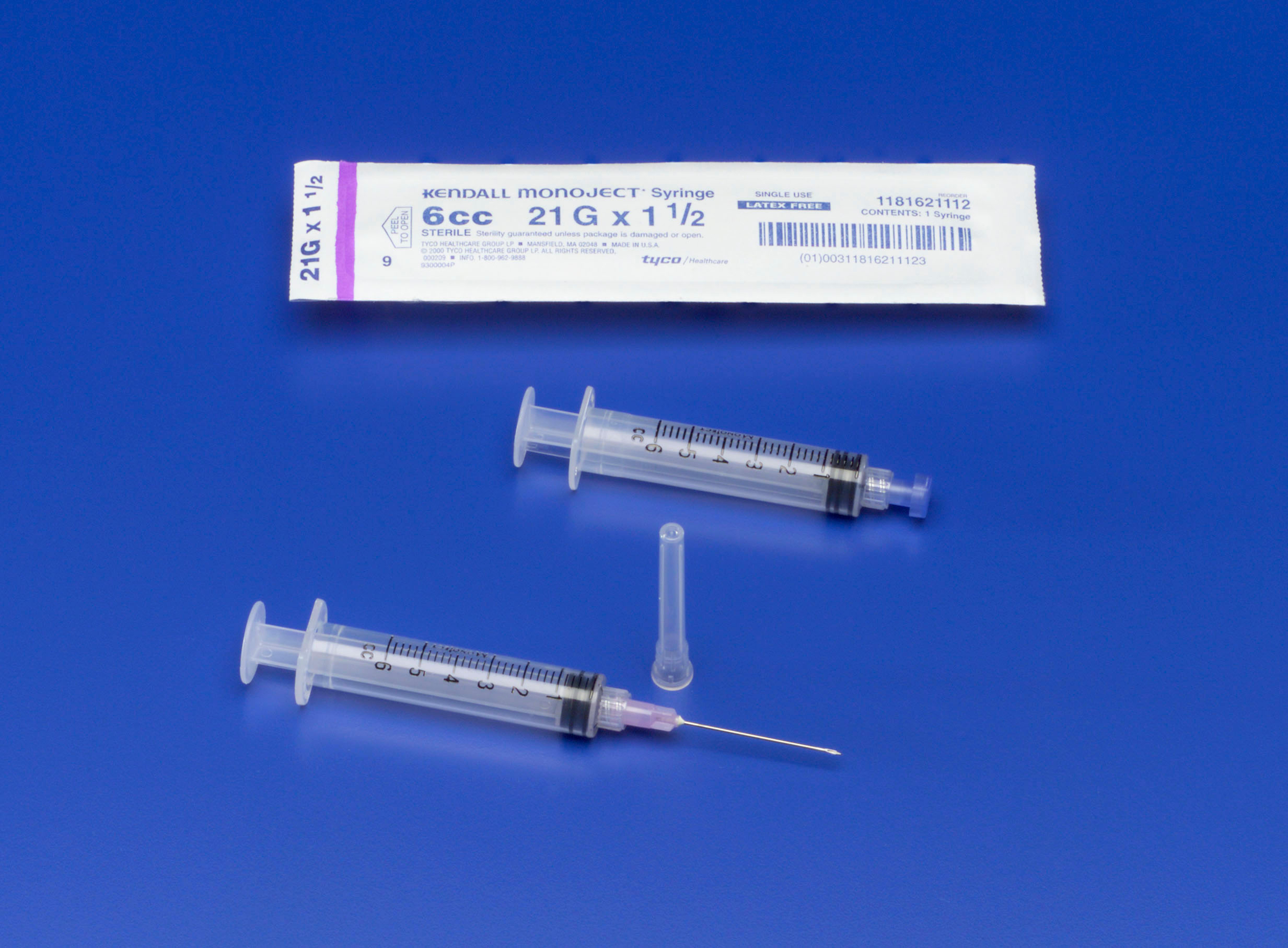
Monoject 1182000777
GUDID 20192253034674
20 mL Syringe Luer-Lock Tip
Cardinal Health 200, LLC
General-purpose syringe, single-use| Primary Device ID | 20192253034674 |
| NIH Device Record Key | 95f0556c-5b38-408a-b7b8-ceed4b8d2c69 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | Monoject |
| Version Model Number | 1182000777 |
| Catalog Number | 1182000777 |
| Company DUNS | 961027315 |
| Company Name | Cardinal Health 200, LLC |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | true |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 10192253034677 [Primary] |
| GS1 | 20192253034674 [Package] Contains: 10192253034677 Package: Carton [40 Units] In Commercial Distribution |
FDA Product Code
| FMF | Syringe, piston |
Sterilization
| Steralize Prior To Use | true |
| Device Is Sterile | true |
[20192253034674]
Radiation Sterilization
[20192253034674]
Radiation Sterilization
[20192253034674]
Radiation Sterilization
[20192253034674]
Radiation Sterilization
[20192253034674]
Radiation Sterilization
[20192253034674]
Radiation Sterilization
[20192253034674]
Radiation Sterilization
[20192253034674]
Radiation Sterilization
[20192253034674]
Radiation Sterilization
[20192253034674]
Radiation Sterilization
[20192253034674]
Radiation Sterilization
[20192253034674]
Radiation Sterilization
[20192253034674]
Radiation Sterilization
[20192253034674]
Radiation Sterilization
[20192253034674]
Radiation Sterilization
[20192253034674]
Radiation Sterilization
[20192253034674]
Radiation Sterilization
[20192253034674]
Radiation Sterilization
[20192253034674]
Radiation Sterilization
[20192253034674]
Radiation Sterilization
[20192253034674]
Radiation Sterilization
[20192253034674]
Radiation Sterilization
[20192253034674]
Radiation Sterilization
[20192253034674]
Radiation Sterilization
[20192253034674]
Radiation Sterilization
[20192253034674]
Radiation Sterilization
[20192253034674]
Radiation Sterilization
[20192253034674]
Radiation Sterilization
[20192253034674]
Radiation Sterilization
[20192253034674]
Radiation Sterilization
[20192253034674]
Radiation Sterilization
[20192253034674]
Radiation Sterilization
[20192253034674]
Radiation Sterilization
[20192253034674]
Radiation Sterilization
[20192253034674]
Radiation Sterilization
[20192253034674]
Radiation Sterilization
[20192253034674]
Radiation Sterilization
[20192253034674]
Radiation Sterilization
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2022-10-31 |
| Device Publish Date | 2022-10-21 |
On-Brand Devices [Monoject]
| 50192253034538 | 1 mL Tuberculin Syringe Luer-Lock Tip |
| 10192253030983 | Monoject™ Oral Dose Syringe Unprinted 10 mL |
| 20192253025313 | Tuberculin Safety Syringe with Needle |
| 10192253025309 | Tuberculin Safety Syringe with Needle |
| 50192253030714 | Control Syringe Luer-Lock Tip |
| 20192253030683 | Control Syringe Luer-Lock Tip |
| 50192253007150 | MONOJECT ENTERAL SYRINGE TIP CAP ENFIT TIP |
| 50192253030943 | Curved Tip Syringes |
| 50192253030738 | 35 mL Pharmacy Tray Luer Lock Tip |
| 50192253030691 | 3 mL Pharmacy Tray Luer Lock Tip |
| 50192253030837 | 140 mL Piston Syringe Catheter Tip |
| 50192253034712 | 60 mL Syringe Catheter Tip |
| 20192253034704 | 35 mL Syringe Catheter Tip |
| 50192253030967 | Monoject 12mL Syringe Luer-Lock Tip |
| 50192253037836 | Monoject™, Luer-Slip Syringe, With Special Plunger, 3 mL, Medtronic Part Number 230570 |
| 50192253011003 | 6 mL Clear Oral Syringe |
| 50192253010990 | 6 mL Amber Oral Syringe |
| 50192253010969 | 1 mL Clear Oral Syringe |
| 20192253025245 | Monoject™ 3 mL Syringe with Hypodermic Safety Needle 25G x 1" (0.5 x 25 mm) |
| 20192253025221 | Monoject™ 3 mL Syringe with Hypodermic Safety Needle 23G x 1" (0.6 x 25 mm) |
| 20192253025207 | Monoject™ 3 mL Syringe with Hypodermic Safety Needle 22G x 1" (0.7 x 25 mm) |
| 20192253025184 | Monoject™ 3 mL Syringe with Hypodermic Safety Needle 21G x 1" (0.8 x 25 mm) |
| 20192253025160 | Monoject™ 3 mL Syringe with Hypodermic Safety Needle 20G x 1" (0.9 x 25 mm) |
| 20192253025153 | Monoject™ 1 mL Syringe with Hypodermic Safety Needle 30G x ¹/2" (0.3 x 13 mm) |
| 20192253025146 | Monoject™ 1 mL Syringe with Hypodermic Safety Needle 27G x ¹/2" (0.4 x 13 mm) |
| 20192253025139 | Monoject™ 1 mL Syringe with Hypodermic Safety Needle 25G x 5/8" (0.5 x 16 mm) |
| 50192253010952 | 1 mL Amber Oral Syringe |
| 20192253025252 | 3 mL Syringe with Hypodermic Safety Needle |
| 20192253025238 | 3 mL Syringe with Hypodermic Safety Needle |
| 20192253025214 | 3 mL Syringe with Hypodermic Safety Needle |
| 20192253025191 | 3 mL Syringe with Hypodermic Safety Needle |
| 20192253025177 | 3 mL Syringe with Hypodermic Safety Needle |
| 50192253038710 | 10 mL Oral Syringe Clear |
| 50192253043653 | Sharps Container Chimney Top, Red 14 Quart |
| 50192253003992 | Sharps Container Chimney Top, Red 8 Quart |
| 50192253003985 | Sharps Container Chimney Top, Red, 4 Quart |
| 20192253034698 | 35 mL Syringe Luer-Lock Tip |
| 20192253034681 | 35 mL Syringe Regular Tip |
| 20192253034674 | 20 mL Syringe Luer-Lock Tip |
| 20192253034667 | 20 mL Syringe Regular Tip |
| 20192253034605 | 6 mL Syringe Luer-Lock Tip |
| 20192253034599 | 6 mL Syringe Regular Tip |
| 20192253033516 | 3 mL Syringe Luer-Lock Tip |
| 20192253033509 | 3 mL Syringe Regular Tip |
| 50192253010983 | 3 mL Oral Syringe Amber |
| 50192253010976 | 3 mL Oral Syringe Clear |
| 50192253005538 | MONOJECT™ GLOVE BOX WITH MOUNTING BRACKET |
| 50192253030950 | 12 mL Syringe Regular Tip |
| 50192253011010 | 10 mL Oral Syringe Amber |
| 50192253063408 | 6 mL Clear Oral Syringe |
Trademark Results [Monoject]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 MONOJECT 74560228 2099750 Dead/Cancelled |
SHERWOOD SERVICES AG 1994-08-08 |
 MONOJECT 74191238 1762511 Dead/Cancelled |
Ecolab Inc. 1991-08-05 |
 MONOJECT 73569258 1403009 Live/Registered |
SHERWOOD MEDICAL COMPANY 1985-11-18 |
 MONOJECT 73569236 1403008 Live/Registered |
SHERWOOD MEDICAL COMPANY 1985-11-18 |
 MONOJECT 73170520 1124445 Dead/Cancelled |
SHERWOOD MEDICAL INDUSTRIES INC. 1978-05-15 |
 MONOJECT 72037194 0664129 Dead/Expired |
ROEHR PRODUCTS CO., INC. 1957-09-13 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.