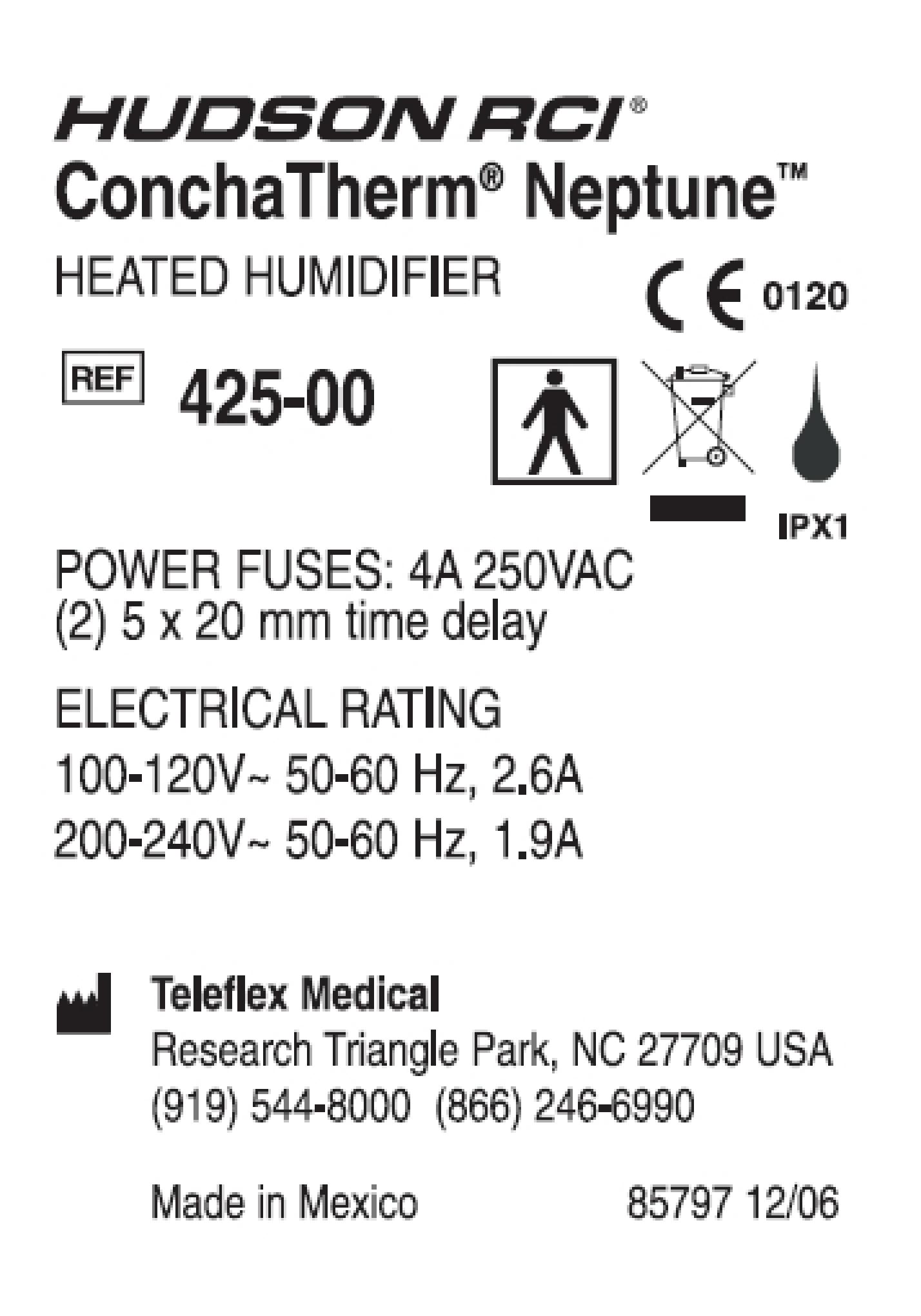
HUDSON RCI HUD717301
GUDID 20197344007698
SPIROMETER,INCENTIVE,TRIFLO II,EXERCISER
MEDLINE INDUSTRIES, INC.
Incentive spirometer| Primary Device ID | 20197344007698 |
| NIH Device Record Key | cd9a903d-2712-4300-92cd-a93d731fa0ac |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | HUDSON RCI |
| Version Model Number | HUD717301 |
| Catalog Number | HUD717301 |
| Company DUNS | 025460908 |
| Company Name | MEDLINE INDUSTRIES, INC. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | true |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | true |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
| Phone | +1(800)633-5463 |
| service@medline.com | |
| Phone | +1(800)633-5463 |
| service@medline.com | |
| Phone | +1(800)633-5463 |
| service@medline.com | |
| Phone | +1(800)633-5463 |
| service@medline.com | |
| Phone | +1(800)633-5463 |
| service@medline.com | |
| Phone | +1(800)633-5463 |
| service@medline.com | |
| Phone | +1(800)633-5463 |
| service@medline.com | |
| Phone | +1(800)633-5463 |
| service@medline.com | |
| Phone | +1(800)633-5463 |
| service@medline.com | |
| Phone | +1(800)633-5463 |
| service@medline.com | |
| Phone | +1(800)633-5463 |
| service@medline.com | |
| Phone | +1(800)633-5463 |
| service@medline.com | |
| Phone | +1(800)633-5463 |
| service@medline.com | |
| Phone | +1(800)633-5463 |
| service@medline.com | |
| Phone | +1(800)633-5463 |
| service@medline.com | |
| Phone | +1(800)633-5463 |
| service@medline.com | |
| Phone | +1(800)633-5463 |
| service@medline.com |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 10197344007691 [Primary] |
| GS1 | 20197344007698 [Package] Contains: 10197344007691 Package: CS [12 Units] In Commercial Distribution |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K220565
FDA Product Code
| BWF | SPIROMETER, THERAPEUTIC (INCENTIVE) |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2023-12-26 |
| Device Publish Date | 2023-12-18 |
On-Brand Devices [HUDSON RCI]
| 10197344007226 | AQUAPAK LVN,STRL WATER,760ML,W/ 33 ADAPT |
| 30197344010275 | O2 CANNULA,PEDIATRIC,SOFTECH,7' TUB,SC |
| 30193489149368 | O2 CANN,ADULT,HI-FLO,EAR CUSH,7 TUB,SC |
| 20193489149354 | O2 CANNULA,ADULT,EAR CUSHION,7' TUB,SC |
| 20197344010094 | O2 CANNULA,INFANT,SOFTECH,7' TUB,SC |
| 20197344009906 | O2 CANNULA,ADULT,SOFTECH,25' TUB,UC |
| 20197344009890 | O2 CANNULA,ADULT,SOFTECH,50' TUB,UC |
| 20197344009883 | O2 CANNULA,PEDIATRIC,SOFTECH,7' TUB,UC |
| 20193489149682 | O2 CANNULA,ADULT,SOFTECH,14' TUB,UC |
| 20193489149347 | O2 CANNULA,ADULT,SOFTECH,7' TUB,SC GREEN |
| 20193489149330 | O2 CANNULA,PEDIATRIC,SOFTECH,14' TUB,SC |
| 20193489149323 | O2 CANNULA,PEDIATRIC,SOFTECH,NO TUBING |
| 20193489149316 | O2 CANNULA,ADULT,SOFTECH,4' TUB,SC |
| 20193489149286 | CANNULA,SOFTECH PLUS,PED,14 GRN STAR-LMN |
| 20193489149279 | O2 CANNULA,PEDI,SOFTECH +,7' TUB,UC |
| 20193489149262 | O2 CANNULA,ADULT,SOFTECH +,7' TUB,UC |
| 10197344007202 | AQUAPAK LVN,STRL WATER,440ML,W/ 28 ADAPT |
| 10193489149241 | O2 CANN,ADULT,HI-FLO,EAR CUSH,14 TUB,SC |
| 20197344010285 | O2 CANNULA,ADULT,SOFTECH,50' TUB,SC |
| 20197344009951 | O2 CANNULA,ADULT,SOFTECH,14' TUB,SC |
| 20197344009937 | O2 CANNULA,ADULT,SOFTECH,NO TUBING |
| 20197344009913 | O2 CANNULA,ADULT,SOFTECH,25' TUB,SC |
| 20197344006028 | AQUAPAK LVN,STRL WATER,1070ML,NO ADAPTER |
| 20193489149699 | O2 CANN,ADULT,SOFTECH,7' SMOOTH TUB,SC |
| 20197344010216 | O2 CANNULA,ADULT,SOFTECH +,7' TUB,SC |
| 20197344010209 | O2 CANNULA,PEDIATRIC,SOFTECH +,7' TUB,SC |
| 20197344010193 | O2 CANNULA,INFANT,SOFTECH +,7' TUB,SC |
| 20197344010186 | O2 CANNULA,NEONATAL,SOFTECH +,7' TUB,SC |
| 20197344010179 | O2 CANNULA,ADULT,SOFTECH +,14' TUB,SC |
| 20197344007698 | SPIROMETER,INCENTIVE,TRIFLO II,EXERCISER |
| 20197344010933 | TEMPERATURE PROBE, DISPOSABLE, NEPTUNE |
| 30197344008128 | ADDIPAK SALINE .45%, 3ML, STERILE |
| 20193489149729 | METER,PEAK FLOW,ASTHMAMD,60-810 LPM |
| 20197344009968 | O2 CANNULA,ADULT,SOFTECH,10' TUB,SC |
| 20197344009944 | O2 CANNULA,ADULT,SOFTECH,7' TUB,SC |
| 20197344009920 | O2 CANNULA,ADULT,SOFTECH,7' TUB,UC |
| 20193489149712 | ADAPTER,AEROSOL,NEB,22MM OD X 18MM OD |
| 20193489149675 | ADAPTR, VALVED TEE, 22MM OD/OD X 18MM |
| 20193489149552 | CANNULA, NASAL, INFANT, COMFORT FLO |
| 10197344037384 | NEBULIZER, TURBOMIST, MOUTHPC, 7' TUB SC |
| 10197344037278 | NEB, TRBMST, PED MSK, 7' TUB, SC, XADPT |
| 20197344007476 | AQUAPAK ADAPTER,ALARM,ADAPTER ONLY |
| 20193489138471 | AQUAKPAK BUBBLE HUM,540ML,NO ADAPTER |
| 10197344037407 | NEB, TURBOMIST, MOUTHPIECE |
| 10197344037391 | NEB, TURBOMIST, MTHPC, 7' TUB SC, XADPT |
| 10197344037292 | NEB, TURBOMIST, ADT MSK, LADT, 7' TUB SC |
| 10197344037285 | NEB, TURBOMIST, ADT MSK, LADT, 7' TUB UC |
| 10197344037261 | NEB, TURBOMIST, PED MSK, LADT, 7' TUB SC |
| 10197344037193 | NEB, TURBOMIST, PED MSK, LADT, 7' TUB UC |
| 20197344077561 | NEB, TURBOMIST, CUP ONLY |
Trademark Results [HUDSON RCI]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 HUDSON RCI 74158130 1695930 Live/Registered |
TELEFLEX MEDICAL INCORPORATED 1991-04-17 |
 HUDSON RCI 74158129 1753651 Dead/Cancelled |
TELEFLEX MEDICAL INCORPORATED 1991-04-17 |
 HUDSON RCI 74157941 1729000 Live/Registered |
TELEFLEX MEDICAL INCORPORATED 1991-04-17 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.