Regard™
GUDID 20326053100974
RESOURCE OPTIMIZATION & INNOVATION, L.L.C.
Neurosurgical procedure kit, non-medicated, single-use| Primary Device ID | 20326053100974 |
| NIH Device Record Key | c43efc67-b841-4341-b268-d32505d0b3cf |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | Regard™ |
| Version Model Number | NU0096 |
| Company DUNS | 945020902 |
| Company Name | RESOURCE OPTIMIZATION & INNOVATION, L.L.C. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | true |
| MRI Safety Status | MR Safe |
| Human Cell/Tissue Product | false |
| Device Kit | true |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 10326053100977 [Primary] |
| GS1 | 20326053100974 [Package] Contains: 10326053100977 Package: [2 Units] Discontinued: 2019-05-31 Not in Commercial Distribution |
| GS1 | 20326053116791 [Package] Contains: 10326053100977 Package: [3 Units] In Commercial Distribution |
FDA Product Code
| OJG | Neurological Tray |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | true |
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 2 |
| Public Version Date | 2019-06-03 |
| Device Publish Date | 2018-02-27 |
On-Brand Devices [Regard™]
Trademark Results [Regard]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
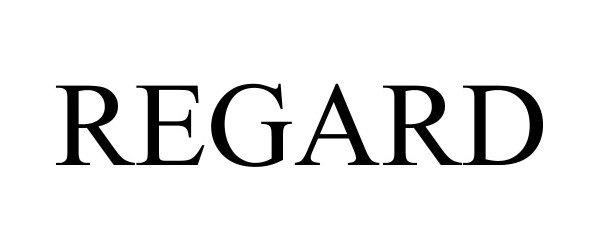 REGARD 97196978 not registered Live/Pending |
HealthTensor, Inc. 2021-12-30 |
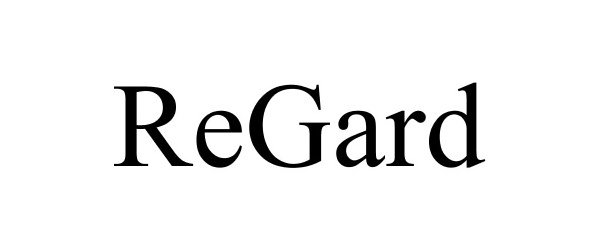 REGARD 86179619 4824780 Live/Registered |
MPC GROUP LLC 2014-01-30 |
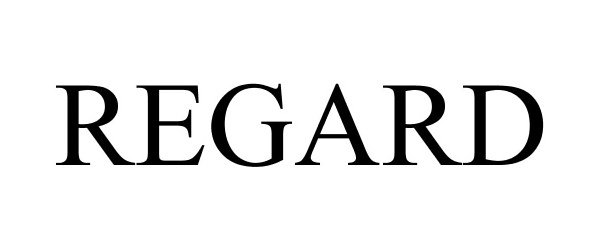 REGARD 86154589 4680759 Live/Registered |
LG Electronics Inc. 2013-12-30 |
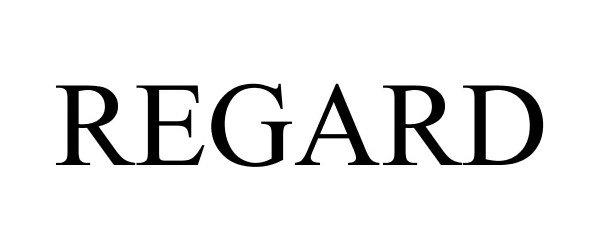 REGARD 85721657 4544205 Live/Registered |
Steelcase Inc. 2012-09-06 |
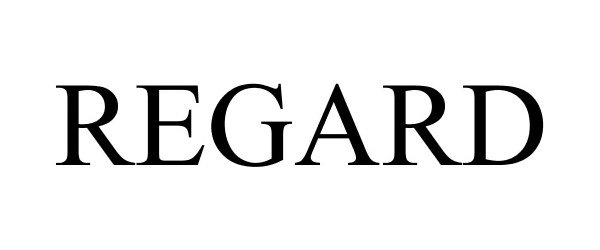 REGARD 85342365 not registered Dead/Abandoned |
Clarke Mosquito Control Products, Inc. 2011-06-09 |
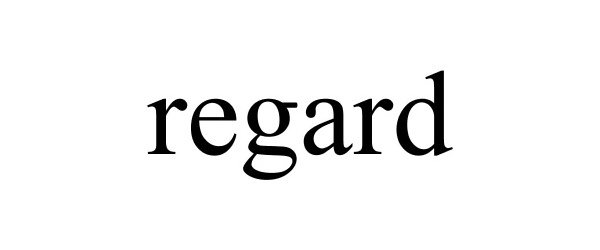 REGARD 85236797 not registered Dead/Abandoned |
M. Z. Berger & Co., Inc. 2011-02-08 |
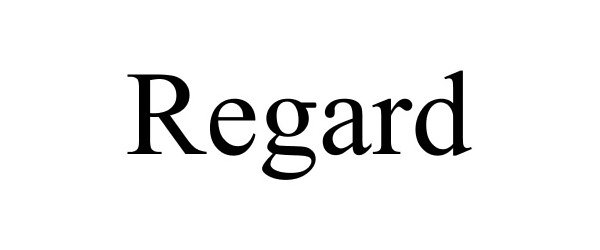 REGARD 85025545 not registered Dead/Abandoned |
Syngenta Participations AG 2010-04-28 |
 REGARD 79033880 3340362 Dead/Cancelled |
Kreon, naamloze vennootschap 2006-12-07 |
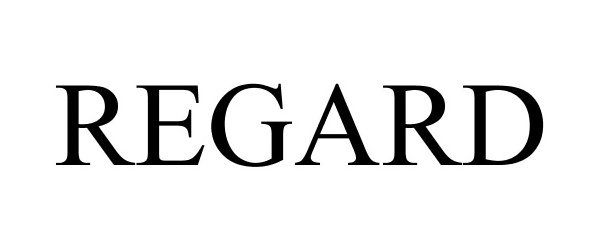 REGARD 78773486 not registered Dead/Abandoned |
Frederick Goldman, Inc. 2005-12-14 |
 REGARD 78064405 2675687 Dead/Cancelled |
Regard Ventures, LLC 2001-05-18 |
 REGARD 77864510 4289323 Live/Registered |
Resource Optimization & Innovation, LLC 2009-11-04 |
 REGARD 75742545 not registered Dead/Abandoned |
GARD CORPORATION 1999-10-30 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.