EXAFLEX® 138305
GUDID 20386040005934
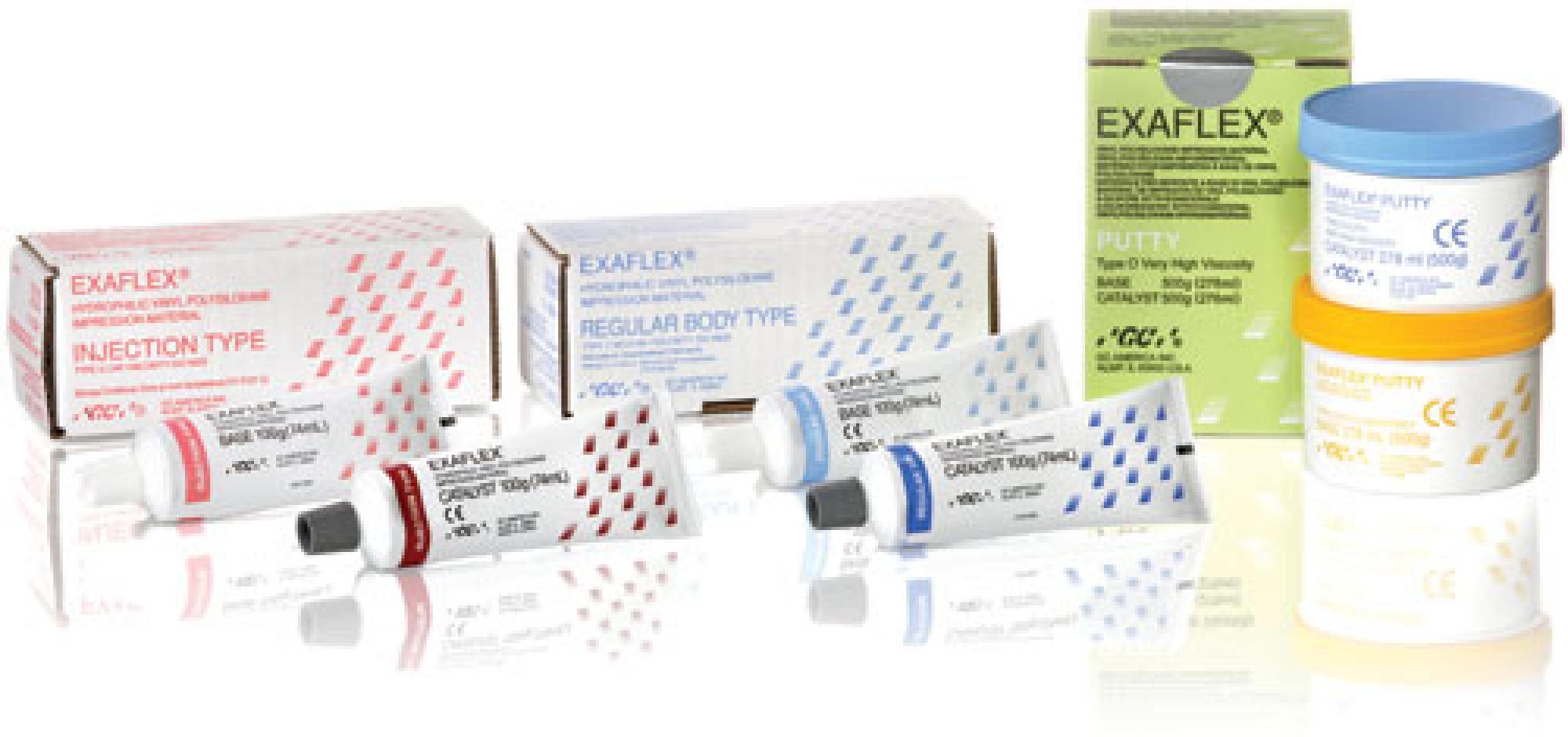
EXAFLEX® 5-5 Pack (5 x 500g blue base Jar, 5 x 500g yellow catalyst Jar, 10 x scoops)
Gc America Inc.
Silicone dental impression material| Primary Device ID | 20386040005934 |
| NIH Device Record Key | 16a8aa2a-ea0d-4984-b8bb-cee4d8500db2 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | EXAFLEX® |
| Version Model Number | 138305 |
| Catalog Number | 138305 |
| Company DUNS | 005473608 |
| Company Name | Gc America Inc. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
| Phone | +1(708)597-0900 |
| customerservice@gcamerica.com | |
| Phone | +1(708)597-0900 |
| customerservice@gcamerica.com | |
| Phone | +1(708)597-0900 |
| customerservice@gcamerica.com | |
| Phone | +1(708)597-0900 |
| customerservice@gcamerica.com | |
| Phone | +1(708)597-0900 |
| customerservice@gcamerica.com | |
| Phone | +1(708)597-0900 |
| customerservice@gcamerica.com | |
| Phone | +1(708)597-0900 |
| customerservice@gcamerica.com | |
| Phone | +1(708)597-0900 |
| customerservice@gcamerica.com | |
| Phone | +1(708)597-0900 |
| customerservice@gcamerica.com |
Operating and Storage Conditions
| Storage Environment Temperature | Between 15 Degrees Celsius and 25 Degrees Celsius |
| Storage Environment Temperature | Between 59 Degrees Fahrenheit and 77 Degrees Fahrenheit |
| Storage Environment Temperature | Between 15 Degrees Celsius and 25 Degrees Celsius |
| Storage Environment Temperature | Between 59 Degrees Fahrenheit and 77 Degrees Fahrenheit |
| Storage Environment Temperature | Between 15 Degrees Celsius and 25 Degrees Celsius |
| Storage Environment Temperature | Between 59 Degrees Fahrenheit and 77 Degrees Fahrenheit |
| Storage Environment Temperature | Between 15 Degrees Celsius and 25 Degrees Celsius |
| Storage Environment Temperature | Between 59 Degrees Fahrenheit and 77 Degrees Fahrenheit |
| Storage Environment Temperature | Between 15 Degrees Celsius and 25 Degrees Celsius |
| Storage Environment Temperature | Between 59 Degrees Fahrenheit and 77 Degrees Fahrenheit |
| Storage Environment Temperature | Between 15 Degrees Celsius and 25 Degrees Celsius |
| Storage Environment Temperature | Between 59 Degrees Fahrenheit and 77 Degrees Fahrenheit |
| Storage Environment Temperature | Between 15 Degrees Celsius and 25 Degrees Celsius |
| Storage Environment Temperature | Between 59 Degrees Fahrenheit and 77 Degrees Fahrenheit |
| Storage Environment Temperature | Between 15 Degrees Celsius and 25 Degrees Celsius |
| Storage Environment Temperature | Between 59 Degrees Fahrenheit and 77 Degrees Fahrenheit |
| Storage Environment Temperature | Between 15 Degrees Celsius and 25 Degrees Celsius |
| Storage Environment Temperature | Between 59 Degrees Fahrenheit and 77 Degrees Fahrenheit |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 10386040005951 [Primary] |
| GS1 | 20386040005934 [Package] Contains: 10386040005951 Package: Carton [6 Units] In Commercial Distribution |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K955932
FDA Product Code
| ELW | MATERIAL, IMPRESSION |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2022-07-15 |
| Device Publish Date | 2022-07-07 |
On-Brand Devices [EXAFLEX®]
| 10386040006040 | EXAFLEX® Regular (blue) Standard Packs (1 x 74mL base, 1 x 74mL catalyst, 1 x mixing pad) |
| 20386040006016 | EXAFLEX® Monophase (purple) Clinic Packs (20 x 74mL base, 20 x 74mL catalyst) |
| 10386040006019 | EXAFLEX® Monophase (purple) Standard Packs (1 x 74mL base, 1 x 74mL catalyst, 1 x mixing pad) |
| 10386040005982 | EXAFLEX® Catalyst (One pail 30 kg) |
| 10386040005975 | EXAFLEX® Base (One pail 30kg) |
| 20386040005934 | EXAFLEX® 5-5 Pack (5 x 500g blue base Jar, 5 x 500g yellow catalyst Jar, 10 x scoops) |
| 10386040005937 | EXAFLEX® Putty 1-1 Pack (1 x 500g blue base Jar, 1 x 500g yellow catalyst Jar, 2 x scoops) |
| 20386040005910 | EXAFLEX® Heavy (yellow) Clinic Packs (20 x 74mL base, 20 x 74mL catalyst) |
| 10386040005913 | EXAFLEX® Heavy (yellow) Standard Packs (1 x 74mL base, 1 x 74mL catalyst, 1 x mixing pad) |
| 20386040005873 | EXAFLEX® Injection (pink) Clinic Packs (20 x 74mL base, 20 x 74mL catalyst) |
| 10386040005876 | EXAFLEX® Injection (pink) Standard Packs (1 x 74mL base, 1 x 74mL catalyst, 1 x mixing pad) |
| 10386040005845 | EXAFLEX® Regular (blue) Clinic Packs (20 x 74mL base, 20 x 74mL catalyst) |
| D6581389012 | EXAFLEX® Regular (blue) Standard Packs (1 x 74mL base, 1 x 74mL catalyst, 1 x mixing pad) |
| D6581384201 | EXAFLEX® Monophase (purple) Clinic Packs (20 x 74mL base, 20 x 74mL catalyst) |
| D6581384012 | EXAFLEX® Monophase (purple) Standard Packs (1 x 74mL base, 1 x 74mL catalyst, 1 x mixing pad) |
| D6581383321 | EXAFLEX® Catalyst (One pail 30 kg) |
| D6581383311 | EXAFLEX® Base (One pail 30kg) |
| D6581383051 | EXAFLEX® 5-5 Pack (5 x 500g blue base Jar, 5 x 500g yellow catalyst Jar, 10 x scoops) |
| D6581383012 | EXAFLEX® Putty 1-1 Pack (1 x 500g blue base Jar, 1 x 500g yellow catalyst Jar, 2 x scoops) |
| D6581382201 | EXAFLEX® Heavy (yellow) Clinic Packs (20 x 74mL base, 20 x 74mL catalyst) |
| D6581382012 | EXAFLEX® Heavy (yellow) Standard Packs (1 x 74mL base, 1 x 74mL catalyst, 1 x mixing pad) |
| D6581381201 | EXAFLEX® Injection (pink) Clinic Packs (20 x 74mL base, 20 x 74mL catalyst) |
| D6581381012 | EXAFLEX® Injection (pink) Standard Packs (1 x 74mL base, 1 x 74mL catalyst, 1 x mixing pad) |
| D6581380201 | EXAFLEX® Regular (blue) Clinic Packs (20 x 74mL base, 20 x 74mL catalyst) |
Trademark Results [EXAFLEX]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 EXAFLEX 90774341 not registered Live/Pending |
Truegenics Pte. Ltd. 2021-06-15 |
 EXAFLEX 73245051 1163378 Live/Registered |
G-C Dental Industrial Corp. 1980-01-03 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.