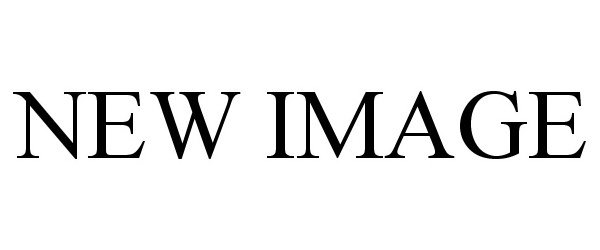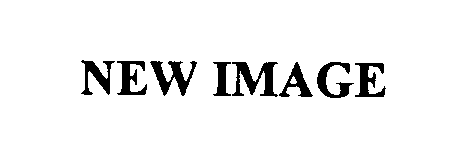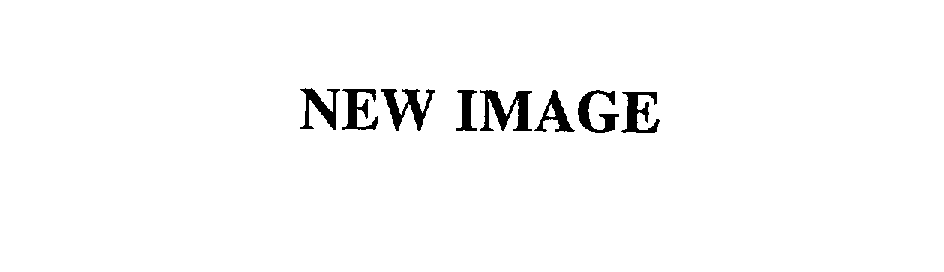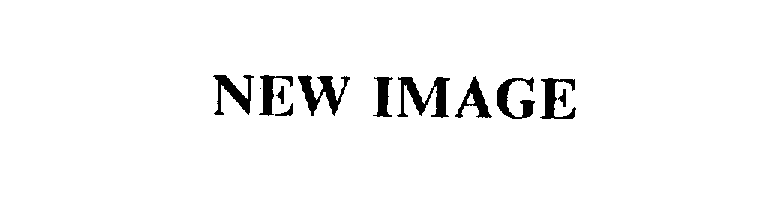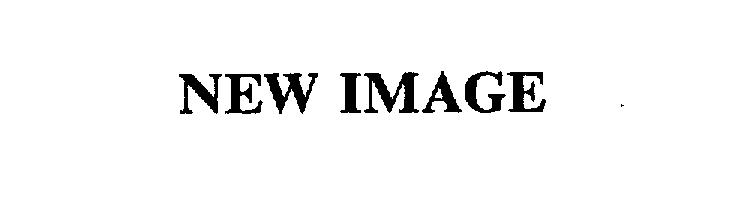New Image 19153
GUDID 20610075036473
Colostomy/Ileostomy Kit,FlexWear
HOLLISTER INCORPORATED
Intestinal ostomy bag/support kit| Primary Device ID | 20610075036473 |
| NIH Device Record Key | a56d697a-711c-411c-8788-ba857aef11be |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | New Image |
| Version Model Number | 19153 |
| Catalog Number | 19153 |
| Company DUNS | 005527098 |
| Company Name | HOLLISTER INCORPORATED |
| Device Count | 5 |
| DM Exempt | false |
| Pre-market Exempt | true |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | true |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | false |
| OTC Over-The-Counter | true |
Customer Support Contacts
| Phone | +1(800)323-4060 |
| cs@hollister.com | |
| Phone | +1(800)323-4060 |
| cs@hollister.com | |
| Phone | +1(800)323-4060 |
| cs@hollister.com | |
| Phone | +1(800)323-4060 |
| cs@hollister.com | |
| Phone | +1(800)323-4060 |
| cs@hollister.com | |
| Phone | +1(800)323-4060 |
| cs@hollister.com | |
| Phone | +1(800)323-4060 |
| cs@hollister.com | |
| Phone | +1(800)323-4060 |
| cs@hollister.com | |
| Phone | +1(800)323-4060 |
| cs@hollister.com | |
| Phone | +1(800)323-4060 |
| cs@hollister.com | |
| Phone | +1(800)323-4060 |
| cs@hollister.com | |
| Phone | +1(800)323-4060 |
| cs@hollister.com |
Device Dimensions
| Lumen/Inner Diameter | 57 Millimeter |
| Lumen/Inner Diameter | 44 Millimeter |
| Lumen/Inner Diameter | 57 Millimeter |
| Lumen/Inner Diameter | 44 Millimeter |
| Lumen/Inner Diameter | 57 Millimeter |
| Lumen/Inner Diameter | 44 Millimeter |
| Lumen/Inner Diameter | 57 Millimeter |
| Lumen/Inner Diameter | 44 Millimeter |
| Lumen/Inner Diameter | 57 Millimeter |
| Lumen/Inner Diameter | 44 Millimeter |
| Lumen/Inner Diameter | 57 Millimeter |
| Lumen/Inner Diameter | 44 Millimeter |
| Lumen/Inner Diameter | 57 Millimeter |
| Lumen/Inner Diameter | 44 Millimeter |
| Lumen/Inner Diameter | 57 Millimeter |
| Lumen/Inner Diameter | 44 Millimeter |
| Lumen/Inner Diameter | 57 Millimeter |
| Lumen/Inner Diameter | 44 Millimeter |
| Lumen/Inner Diameter | 57 Millimeter |
| Lumen/Inner Diameter | 44 Millimeter |
| Lumen/Inner Diameter | 57 Millimeter |
| Lumen/Inner Diameter | 44 Millimeter |
| Lumen/Inner Diameter | 57 Millimeter |
| Lumen/Inner Diameter | 44 Millimeter |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00610075114924 [Unit of Use] |
| GS1 | 10610075114129 [Primary] |
| GS1 | 20610075036473 [Package] Contains: 10610075114129 Package: CAS [6 Units] In Commercial Distribution |
FDA Product Code
| PQE | Ostomy care kit |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | true |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2022-12-09 |
| Device Publish Date | 2022-12-01 |
On-Brand Devices [New Image]
| 10610075115041 | Two-Piece Mini Drainable Pre-sized Pouch, with Filter, Lock 'n Roll closure, Transparent, 70 mm, |
| 20610075114140 | Sterile Kit with Drainable Pouch, Clamp Closure, Loop Ostomy Bridge |
| 20610075114119 | Sterile Kit with Drainable Pouch, Lock 'n Roll Closure, Loop Ostomy Bridge |
| 20610075114089 | Non-sterile Kit with Drainable Pouch, Clamp Closure, Loop Ostomy Bridge |
| 20610075114058 | Non-sterile Kit with Drainable Pouch, Lock 'n Roll Closure, Loop Ostomy Bridge |
| 20610075198638 | Urostomy Kit, Flextend |
| 20610075198621 | Colostomy/Ileostomy Kit, FlexWear |
| 20610075198614 | Colostomy/Ileostomy Kit, FlexWear |
| 20610075186321 | Urostomy Kit, FormaFlex |
| 20610075186307 | Urostomy Kit, FormaFlex |
| 20610075186284 | Colostomy/Ileostomy Kit,FormaFlex |
| 20610075186260 | Colostomy/Ileostomy Kit,FormaFlex |
| 20610075036572 | Urostomy Kit, Flextend |
| 20610075036466 | Colostomy/Ileostomy Kit,FlexWear |
| 20610075036244 | Colostomy/Ileostomy Kit,FlexWear |
| 20610075132342 | Urostomy Kit, Flextend |
| 20610075036282 | Colostomy/Ileostomy Kit, FlexWear |
| 20610075036206 | Colostomy/Ileostomy Kit, FlexWear |
| 20610075114744 | 2-Piece Drainable Ostomy Pouch with Soft Tap |
| 20610075036565 | Urostomy Kit, Flextend |
| 20610075036558 | Urostomy Kit, Flextend |
| 20610075036534 | Urostomy Kit, Flextend |
| 20610075036527 | Urostomy Kit, Flextend |
| 20610075036503 | Colostomy/Ileostomy Kit,FlexWear |
| 20610075036473 | Colostomy/Ileostomy Kit,FlexWear |
| 20610075036268 | Colostomy/Ileostomy Kit,FlexWear |
| 20610075036251 | Colostomy/Ileostomy Kit,FlexWear |
| 20610075036404 | Colostomy/Ileostomy Kit, FlexWear |
| 20610075036299 | Colostomy/Ileostomy Kit, FlexWear |
| 20610075036220 | Colostomy/Ileostomy Kit, FlexWear |
| 20610075036213 | Colostomy/Ileostomy Kit, FlexWear |
| 20610075187977 | 2-Piece Urostomy Pouch |
| 20610075187960 | 2-Piece Urostomy Pouch |
| 20610075187953 | 2-Piece Urostomy Pouch |
| 20610075187946 | 2-Piece Urostomy Pouch |
| 20610075187939 | 2-Piece Urostomy Pouch |
| 20610075187922 | 2-Piece Urostomy Pouch |
| 20610075187915 | 2-Piece Urostomy Pouch |
| 20610075187908 | 2-Piece Urostomy Pouch |
| 20610075187892 | 2-Piece Urostomy Pouch |
| 20610075184143 | 2-Piece Urostomy Pouch |
| 20610075184136 | 2-Piece Urostomy Pouch |
| 20610075184129 | 2-Piece Urostomy Pouch |
| 20610075184044 | 2-Piece Urostomy Pouch |
| 20610075184037 | 2-Piece Urostomy Pouch |
| 20610075184020 | 2-Piece Urostomy Pouch |
| 20610075182040 | 2-Piece Drainable Ostomy Pouch |
| 20610075182033 | 2-Piece Drainable Ostomy Pouch |
| 20610075182026 | 2-Piece Drainable Ostomy Pouch |
| 20610075181944 | 2-Piece Drainable Ostomy Pouch |
Trademark Results [New Image]
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.