INPUT™ TS
GUDID 20643169014415
INTRODUCER 040028A 5F 7CM ENH INSERT TS
MEDTRONIC, INC.
Vascular catheter introduction set, nonimplantable| Primary Device ID | 20643169014415 |
| NIH Device Record Key | 44ab911c-540a-4afb-a8e4-05c894730085 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | INPUT™ TS |
| Version Model Number | 040028A |
| Company DUNS | 006261481 |
| Company Name | MEDTRONIC, INC. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
| Phone | +1(800)633-8766 |
| Corporate.UDI@medtronic.com | |
| Phone | +1(800)633-8766 |
| Corporate.UDI@medtronic.com | |
| Phone | +1(800)633-8766 |
| Corporate.UDI@medtronic.com | |
| Phone | +1(800)633-8766 |
| Corporate.UDI@medtronic.com | |
| Phone | +1(800)633-8766 |
| Corporate.UDI@medtronic.com | |
| Phone | +1(800)633-8766 |
| Corporate.UDI@medtronic.com | |
| Phone | +1(800)633-8766 |
| Corporate.UDI@medtronic.com | |
| Phone | +1(800)633-8766 |
| Corporate.UDI@medtronic.com | |
| Phone | +1(800)633-8766 |
| Corporate.UDI@medtronic.com | |
| Phone | +1(800)633-8766 |
| Corporate.UDI@medtronic.com | |
| Phone | +1(800)633-8766 |
| Corporate.UDI@medtronic.com | |
| Phone | +1(800)633-8766 |
| Corporate.UDI@medtronic.com | |
| Phone | +1(800)633-8766 |
| Corporate.UDI@medtronic.com | |
| Phone | +1(800)633-8766 |
| Corporate.UDI@medtronic.com | |
| Phone | +1(800)633-8766 |
| Corporate.UDI@medtronic.com | |
| Phone | +1(800)633-8766 |
| Corporate.UDI@medtronic.com | |
| Phone | +1(800)633-8766 |
| Corporate.UDI@medtronic.com | |
| Phone | +1(800)633-8766 |
| Corporate.UDI@medtronic.com | |
| Phone | +1(800)633-8766 |
| Corporate.UDI@medtronic.com |
Device Dimensions
| Length | 7 Centimeter |
| Length | 7 Centimeter |
| Length | 7 Centimeter |
| Length | 7 Centimeter |
| Length | 7 Centimeter |
| Length | 7 Centimeter |
| Catheter Gauge | 5 French |
| Outer Diameter | 0.97 Millimeter |
| Length | 7 Centimeter |
| Catheter Gauge | 5 French |
| Outer Diameter | 0.97 Millimeter |
| Length | 7 Centimeter |
| Catheter Gauge | 5 French |
| Outer Diameter | 0.97 Millimeter |
| Length | 7 Centimeter |
| Catheter Gauge | 5 French |
| Outer Diameter | 0.97 Millimeter |
| Length | 7 Centimeter |
| Catheter Gauge | 5 French |
| Outer Diameter | 0.97 Millimeter |
| Length | 7 Centimeter |
| Catheter Gauge | 5 French |
| Outer Diameter | 0.97 Millimeter |
| Length | 7 Centimeter |
| Catheter Gauge | 5 French |
| Outer Diameter | 0.97 Millimeter |
| Length | 7 Centimeter |
| Catheter Gauge | 5 French |
| Outer Diameter | 0.97 Millimeter |
| Length | 7 Centimeter |
| Catheter Gauge | 5 French |
| Outer Diameter | 0.97 Millimeter |
| Length | 7 Centimeter |
| Catheter Gauge | 5 French |
| Outer Diameter | 0.97 Millimeter |
| Length | 7 Centimeter |
| Catheter Gauge | 5 French |
| Outer Diameter | 0.97 Millimeter |
| Length | 7 Centimeter |
| Catheter Gauge | 5 French |
| Outer Diameter | 0.97 Millimeter |
| Length | 7 Centimeter |
| Catheter Gauge | 5 French |
| Outer Diameter | 0.97 Millimeter |
| Length | 7 Centimeter |
| Catheter Gauge | 5 French |
| Outer Diameter | 0.97 Millimeter |
| Length | 7 Centimeter |
| Catheter Gauge | 5 French |
| Outer Diameter | 0.97 Millimeter |
| Length | 7 Centimeter |
| Catheter Gauge | 5 French |
| Outer Diameter | 0.97 Millimeter |
| Length | 7 Centimeter |
| Catheter Gauge | 5 French |
| Outer Diameter | 0.97 Millimeter |
| Length | 7 Centimeter |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00643169014411 [Primary] |
| GS1 | 20643169014415 [Package] Contains: 00643169014411 Package: PK [5 Units] In Commercial Distribution |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K955785
FDA Product Code
| DYB | INTRODUCER, CATHETER |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | true |
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 4 |
| Public Version Date | 2019-06-07 |
| Device Publish Date | 2017-02-02 |
On-Brand Devices [INPUT™ TS]
| 20643169014637 | INTRODUCER 060037A 6F 23CM ENH INSERT TS |
| 20643169014606 | INTRODUCER 061101A 6F 11CM ENH INSERT TS |
| 20643169014590 | INTRDCR 061102A 5PK 11CM 6F INPT TS WNDL |
| 20643169014569 | INTRODUCER 070037A 7F 23CM ENH INSERT TS |
| 20643169014538 | INTRODUCER 071101A 7F 11CM ENH INSERT TS |
| 20643169014521 | INTRDCR 071102A 5PK 11CM 7F INPT TS WNDL |
| 20643169014491 | INTRODUCER 080037A 8F 23CM ENH INSERT TS |
| 20643169014460 | INTRODUCER 081101A 8F 11CM ENH INSERT TS |
| 20643169014453 | INTRDCR 081102A 5PK 11CM 8F INPT TS WNDL |
| 20643169014446 | INTRDCR 051102A 11CM 5F INPT TS W NL 5PK |
| 20643169014439 | INTRODUCER 010101A 10F 11CM ENH INSRT TS |
| 20643169014422 | INTRDCR 011101A 5PK 11F 11CM ENH INST TS |
| 20643169014415 | INTRODUCER 040028A 5F 7CM ENH INSERT TS |
| 20643169014408 | INTRODUCER 040031A 6F 7CM ENH INSERT TS |
| 20643169014392 | INTRODUCER 040035A 7F 7CM ENH INSERT TS |
| 20643169014309 | INTRODUCER 051101A 5F 11CM ENH INSERT TS |
| 20643169014095 | INTRDCR 090037A 5PK 9F 23CM ENH INSRT TS |
| 20643169014088 | INTRODUCER 091101A 9F 11CM ENH INSERT TS |
| 20643169014071 | INTRDCR 091102A 5PK 11CM 9F INPT TS WNDL |
Trademark Results [INPUT]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 INPUT 97447940 not registered Live/Pending |
El-Ghawaby, Mohab 2022-06-08 |
 INPUT 88524916 not registered Live/Pending |
BDG Media, Inc. 2019-07-19 |
 INPUT 87978649 5603411 Live/Registered |
Input Tech, Inc. 2017-02-24 |
 INPUT 87349048 not registered Live/Pending |
Input Tech, Inc. 2017-02-24 |
 INPUT 87017908 not registered Dead/Abandoned |
Peter Cunningham 2016-04-28 |
 INPUT 86217526 4617376 Live/Registered |
THE FONT BUREAU INC. 2014-03-11 |
 INPUT 79289760 not registered Live/Pending |
SEI-HEI MOON, S.L. 2020-06-19 |
 INPUT 78203132 2788957 Dead/Cancelled |
INPUT, INC. 2003-01-14 |
 INPUT 77770848 3990598 Dead/Cancelled |
Krueger International, Inc. 2009-06-30 |
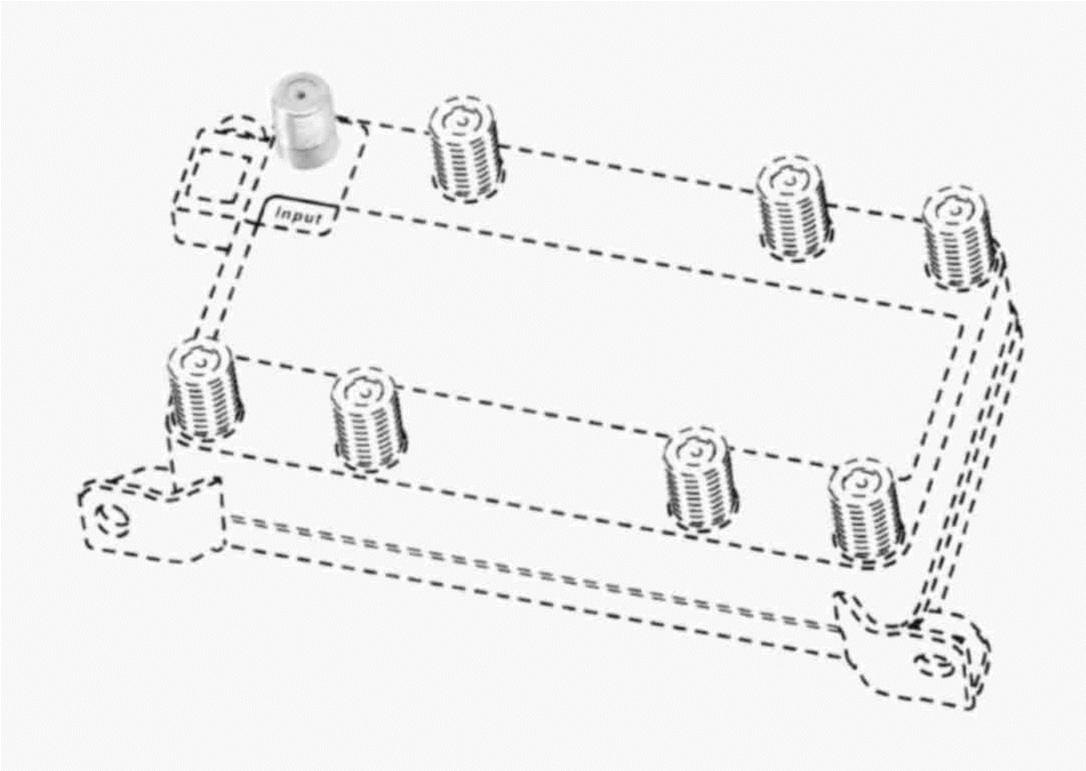 INPUT 76714207 4479134 Live/Registered |
TIMES FIBER COMMUNICATIONS, INC. 2013-05-23 |
 INPUT 76188467 2946849 Live/Registered |
Gallup, Inc. 2001-01-02 |
 INPUT 75605621 2468218 Dead/Cancelled |
Punch Products USA, Inc. 1998-12-14 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.