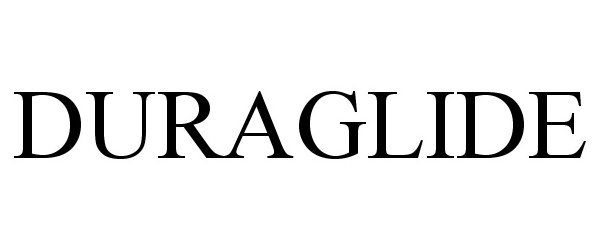DURAGLIDE 007143
GUDID 20653405053733
STONE BLN 3L,8.5MM,BELOW
Conmed Corporation
Biliary stone retrieval balloon catheter| Primary Device ID | 20653405053733 |
| NIH Device Record Key | e5d79b7f-fc94-4013-855b-c5c790d41882 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | DURAGLIDE |
| Version Model Number | 007143 |
| Catalog Number | 007143 |
| Company DUNS | 071595540 |
| Company Name | Conmed Corporation |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
Device Dimensions
| Outer Diameter | 8.5 Millimeter |
| Outer Diameter | 8.5 Millimeter |
| Outer Diameter | 8.5 Millimeter |
| Outer Diameter | 8.5 Millimeter |
| Outer Diameter | 8.5 Millimeter |
| Outer Diameter | 8.5 Millimeter |
| Outer Diameter | 8.5 Millimeter |
| Outer Diameter | 8.5 Millimeter |
| Outer Diameter | 8.5 Millimeter |
| Outer Diameter | 8.5 Millimeter |
| Outer Diameter | 8.5 Millimeter |
| Outer Diameter | 8.5 Millimeter |
| Outer Diameter | 8.5 Millimeter |
| Outer Diameter | 8.5 Millimeter |
| Outer Diameter | 8.5 Millimeter |
| Outer Diameter | 8.5 Millimeter |
| Outer Diameter | 8.5 Millimeter |
| Outer Diameter | 8.5 Millimeter |
| Outer Diameter | 8.5 Millimeter |
| Outer Diameter | 8.5 Millimeter |
| Outer Diameter | 8.5 Millimeter |
| Outer Diameter | 8.5 Millimeter |
| Outer Diameter | 8.5 Millimeter |
| Outer Diameter | 8.5 Millimeter |
| Outer Diameter | 8.5 Millimeter |
| Outer Diameter | 8.5 Millimeter |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00653405053739 [Previous] |
| GS1 | 20653405053733 [Primary] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K993892
FDA Product Code
| FGE | Stents, drains and dilators for the biliary ducts |
Sterilization
| Steralize Prior To Use | true |
| Device Is Sterile | true |
[20653405053733]
Ethylene Oxide
[20653405053733]
Ethylene Oxide
[20653405053733]
Ethylene Oxide
[20653405053733]
Ethylene Oxide
[20653405053733]
Ethylene Oxide
[20653405053733]
Ethylene Oxide
[20653405053733]
Ethylene Oxide
[20653405053733]
Ethylene Oxide
[20653405053733]
Ethylene Oxide
[20653405053733]
Ethylene Oxide
[20653405053733]
Ethylene Oxide
[20653405053733]
Ethylene Oxide
[20653405053733]
Ethylene Oxide
[20653405053733]
Ethylene Oxide
[20653405053733]
Ethylene Oxide
[20653405053733]
Ethylene Oxide
[20653405053733]
Ethylene Oxide
[20653405053733]
Ethylene Oxide
[20653405053733]
Ethylene Oxide
[20653405053733]
Ethylene Oxide
[20653405053733]
Ethylene Oxide
[20653405053733]
Ethylene Oxide
[20653405053733]
Ethylene Oxide
[20653405053733]
Ethylene Oxide
[20653405053733]
Ethylene Oxide
[20653405053733]
Ethylene Oxide
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2020-10-26 |
| Device Publish Date | 2020-10-16 |
On-Brand Devices [DURAGLIDE]
| 00653405053753 | DURAGLIDE STONE BALLOON 15MM, BELOW |
| 00653405053746 | DURAGLIDE STONE BALLOON 11.5MM, BELOW |
| 00653405053739 | DURAGLIDE STONE BALLOON 8.5MM, BELOW |
| 00653405053722 | DURAGLIDE STONE BALLOON 15MM, ABOVE |
| 00653405053715 | DURAGLIDE STONE BALLOON 11.5MM, ABOVE |
| 00653405053708 | DURAGLIDE STONE BALLOON 8.5MM, ABOVE |
| 20653405053801 | DURAGLIDE Stone Balloon, 15.0mm, 7-5f |
| 20653405053795 | DURAGLIDE Stone Balloon, 11.5mm, 7-5f |
| 20653405053788 | DURAGLIDE Stone Balloon, 8.5mm, 7-5f |
| 20653405053771 | DURAGLIDE Stone Balloon, 15.0mm, 7f |
| 20653405053764 | DURAGLIDE Stone Balloon, 11.5mm, 7f |
| 20653405053757 | STONE BLN 3L,15MM BELOW |
| 20653405053733 | STONE BLN 3L,8.5MM,BELOW |
Trademark Results [DURAGLIDE]
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.