Donaldson
GUDID 20681490028305
VENT TUBE 1012015 5PK DONALDSON W/MEMBR
MEDTRONIC XOMED, INC.
Tympanostomy tube| Primary Device ID | 20681490028305 |
| NIH Device Record Key | db612c16-51e1-4561-82d4-c460a6f62e52 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | Donaldson |
| Version Model Number | 1012015 |
| Company DUNS | 835465063 |
| Company Name | MEDTRONIC XOMED, INC. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | MR Conditional |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
Device Dimensions
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00681490028301 [Primary] |
| GS1 | 20681490028305 [Package] Contains: 00681490028301 Package: PK [5 Units] In Commercial Distribution |
FDA Product Code
| ETD | TUBE, TYMPANOSTOMY |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | true |
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 2 |
| Public Version Date | 2018-03-29 |
| Device Publish Date | 2015-07-13 |
On-Brand Devices [Donaldson]
| 20613994176701 | VENT TUBE 1015102 10PK DNLDSN PR 1.14 FP |
| 20613994176589 | VENT TUBE 1015201 5PK DLDSN WIRE 1.14 FP |
| 20613994176572 | VENT TUBE 1015101 5PK DONLDSN 1.14 FLPL |
| 20681490306779 | VENT TUBE MV1025 10PK DONALDSON 1.14 SIL |
| 20681490062095 | VENT TUBE 24161 5PK DONALDSON FIRM 1.14 |
| 20681490062088 | VENT TUBE 24152 10PK DONLDSN FIRM SIL PR |
| 20681490062071 | VENT TUBE 24151 5PK DONALD 1.14 FIRM SIL |
| 20681490031787 | VENT TUBE 1056010 5PK DONALDSON 1.14MM |
| 20681490031367 | VENT TUBE 1046020 10PK DONALDSON PAIRS |
| 20681490031091 | VENT TUBE 1040009 10PK DONALDSON PR 1.14 |
| 20681490030520 | VENT TUBE 1033000 ACTIVENT 5PK DONALDSON |
| 20681490028381 | VENT TUBE 1013021 50PK DONALDSON |
| 20681490028374 | VENT TUBE 1013020 5PK DONALD 1.14MM SIL |
| 20681490028367 | VENT TUBE 1013015 5PK DONALD W/TAB SILIC |
| 20681490028305 | VENT TUBE 1012015 5PK DONALDSON W/MEMBR |
| 20763000037653 | VENT TUBE 1015102 10PK DNLDSN PR 1.14 FP |
| 20763000037578 | VENT TUBE 1015101 5PK DONLDSN 1.14 FLPL |
| 20763000038599 | VENT TUBE 1013021 50PK DONALDSON |
| 20763000038582 | VENT TUBE 1013020 5PK DONALD 1.14MM SIL |
| 20763000038575 | VENT TUBE 1013015 5PK DONALD W/TAB SILIC |
| 20763000040912 | VENT TUBE MV1025 10PK DONALDSON 1.14 SIL |
| 20763000870540 | VENT TUBE 1013020 5PK DONALD 1.14MM SIL |
| 20763000870533 | VENT TUBE 1013015 5PK DONALD W/TAB SILIC |
| 20763000870410 | VENT TUBE 1015101 5PK DONLDSN 1.14 FLPL |
Trademark Results [Donaldson]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
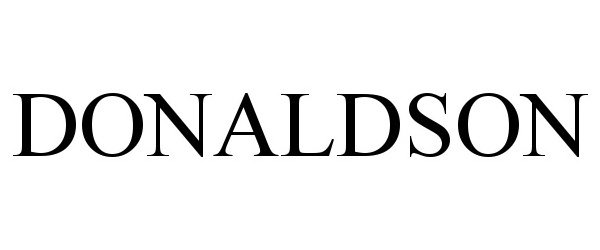 DONALDSON 90815594 not registered Live/Pending |
Donaldson Company, Inc. 2021-07-07 |
 DONALDSON 81025509 1025509 Dead/Cancelled |
Oregon Aqua-Foods, Inc. 0000-00-00 |
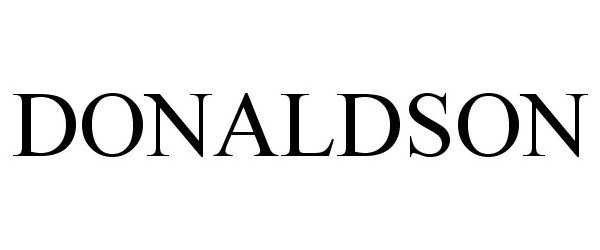 DONALDSON 77327288 3487750 Live/Registered |
DONALDSON INTERIORS, INC. 2007-11-12 |
 DONALDSON 74457350 1947900 Dead/Cancelled |
BLUE PRODUCTION 1993-11-08 |
 DONALDSON 74457334 1911474 Dead/Cancelled |
BLUE PRODUCTION 1993-11-08 |
 DONALDSON 74457333 1908533 Dead/Cancelled |
BLUE PRODUCTION 1993-11-08 |
 DONALDSON 74457332 1947899 Dead/Cancelled |
BLUE PRODUCTION 1993-11-08 |
 DONALDSON 74253557 1765405 Dead/Cancelled |
BLUE PRODUCTION 1992-03-09 |
 DONALDSON 73469086 1357629 Live/Registered |
DONALDSON COMPANY, INC. 1984-03-07 |
 DONALDSON 72341311 0902587 Live/Registered |
DONALDSON COMPANY, INC. 1969-10-22 |
 DONALDSON 72271776 0865273 Live/Registered |
DONALDSON COMPANY, INC. 1967-05-18 |
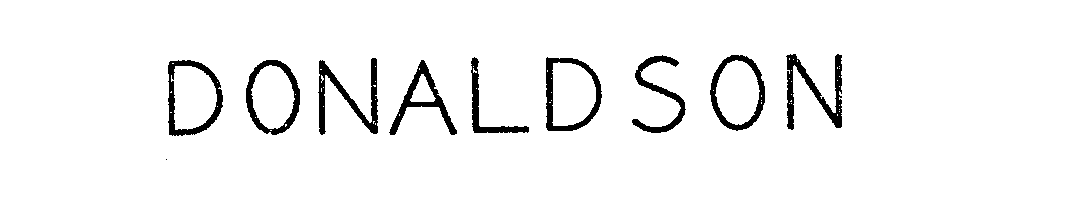 DONALDSON 71677398 0620305 Live/Registered |
DONALDSON COMPANY, INC. 1954-11-29 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.