IsoLoc ISO-60
GUDID 20851546007213
IsoLoc Short Profile Prostate Immobilization Treatment Balloon Device Kit
RADIADYNE, L.L.C.
Rectosphincteric balloon| Primary Device ID | 20851546007213 |
| NIH Device Record Key | 58fc5ac2-3a18-4d16-b7f2-fd5f0ce3d738 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | IsoLoc |
| Version Model Number | ISO-60 |
| Catalog Number | ISO-60 |
| Company DUNS | 005744772 |
| Company Name | RADIADYNE, L.L.C. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | MR Conditional |
| Human Cell/Tissue Product | false |
| Device Kit | true |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | true |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
Operating and Storage Conditions
| Storage Environment Temperature | Between -30 Degrees Celsius and 50 Degrees Celsius |
| Storage Environment Temperature | Between -30 Degrees Celsius and 50 Degrees Celsius |
| Storage Environment Temperature | Between -30 Degrees Celsius and 50 Degrees Celsius |
| Storage Environment Temperature | Between -30 Degrees Celsius and 50 Degrees Celsius |
| Storage Environment Temperature | Between -30 Degrees Celsius and 50 Degrees Celsius |
| Storage Environment Temperature | Between -30 Degrees Celsius and 50 Degrees Celsius |
| Storage Environment Temperature | Between -30 Degrees Celsius and 50 Degrees Celsius |
| Storage Environment Temperature | Between -30 Degrees Celsius and 50 Degrees Celsius |
| Storage Environment Temperature | Between -30 Degrees Celsius and 50 Degrees Celsius |
| Storage Environment Temperature | Between -30 Degrees Celsius and 50 Degrees Celsius |
| Storage Environment Temperature | Between -30 Degrees Celsius and 50 Degrees Celsius |
| Storage Environment Temperature | Between -30 Degrees Celsius and 50 Degrees Celsius |
| Storage Environment Temperature | Between -30 Degrees Celsius and 50 Degrees Celsius |
| Storage Environment Temperature | Between -30 Degrees Celsius and 50 Degrees Celsius |
| Storage Environment Temperature | Between -30 Degrees Celsius and 50 Degrees Celsius |
| Storage Environment Temperature | Between -30 Degrees Celsius and 50 Degrees Celsius |
| Storage Environment Temperature | Between -30 Degrees Celsius and 50 Degrees Celsius |
| Storage Environment Temperature | Between -30 Degrees Celsius and 50 Degrees Celsius |
| Storage Environment Temperature | Between -30 Degrees Celsius and 50 Degrees Celsius |
| Storage Environment Temperature | Between -30 Degrees Celsius and 50 Degrees Celsius |
| Storage Environment Temperature | Between -30 Degrees Celsius and 50 Degrees Celsius |
| Storage Environment Temperature | Between -30 Degrees Celsius and 50 Degrees Celsius |
| Storage Environment Temperature | Between -30 Degrees Celsius and 50 Degrees Celsius |
| Storage Environment Temperature | Between -30 Degrees Celsius and 50 Degrees Celsius |
| Storage Environment Temperature | Between -30 Degrees Celsius and 50 Degrees Celsius |
| Storage Environment Temperature | Between -30 Degrees Celsius and 50 Degrees Celsius |
| Storage Environment Temperature | Between -30 Degrees Celsius and 50 Degrees Celsius |
| Storage Environment Temperature | Between -30 Degrees Celsius and 50 Degrees Celsius |
| Storage Environment Temperature | Between -30 Degrees Celsius and 50 Degrees Celsius |
| Storage Environment Temperature | Between -30 Degrees Celsius and 50 Degrees Celsius |
| Storage Environment Temperature | Between -30 Degrees Celsius and 50 Degrees Celsius |
| Storage Environment Temperature | Between -30 Degrees Celsius and 50 Degrees Celsius |
| Storage Environment Temperature | Between -30 Degrees Celsius and 50 Degrees Celsius |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00851546007219 [Primary] |
| GS1 | 10851546007216 [Package] Contains: 00851546007219 Package: Shelf Box [20 Units] In Commercial Distribution |
| GS1 | 20851546007213 [Package] Contains: 10851546007216 Package: Shipper Box [5 Units] In Commercial Distribution |
FDA Pre-market Approvals/Notifications & deNovo
- deNovo: DEN130036
FDA Product Code
| PCT | Prostate Immobilizer Rectal Balloon |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 4 |
| Public Version Date | 2018-07-27 |
| Device Publish Date | 2018-01-31 |
On-Brand Devices [IsoLoc]
| 20851546007213 | IsoLoc Short Profile Prostate Immobilization Treatment Balloon Device Kit |
| 20851546007107 | IsoLoc Prostate Immobilization Treatment Balloon Device Kit |
| 00851546007092 | IsoLoc Short Profile Prostate Immobilization Treatment Balloon Device Kit |
| 20851546007091 | IsoLoc Short Profile Prostate Immobilization Treatment Balloon Device Kit |
Trademark Results [IsoLoc]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
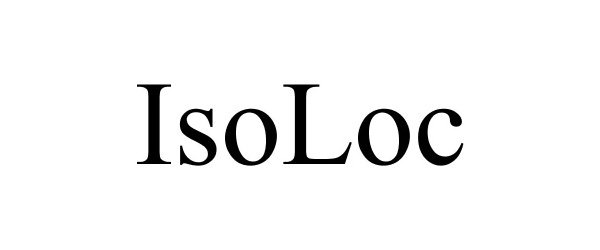 ISOLOC 88086550 5714724 Live/Registered |
ANGIODYNAMICS, INC. 2018-08-21 |
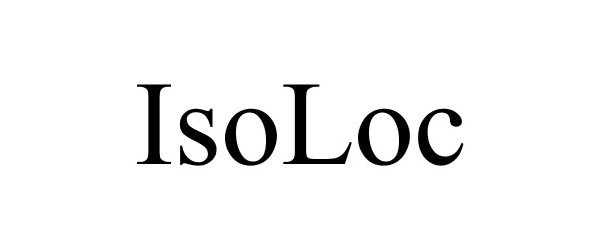 ISOLOC 77571441 not registered Dead/Abandoned |
Isham, John 2008-09-16 |
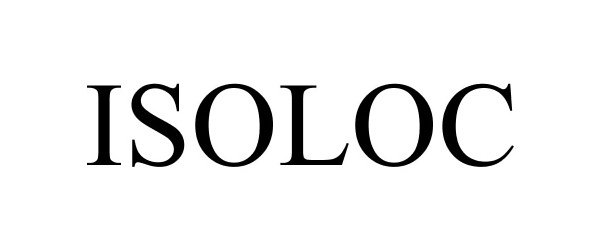 ISOLOC 77568678 not registered Dead/Abandoned |
Stabiloc, LLC 2008-09-12 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.