MYNXGRIP MX6721
GUDID 20862028000417
MynxGrip 6F/7F
Access Closure, Inc.
Wound hydrogel dressing, sterile| Primary Device ID | 20862028000417 |
| NIH Device Record Key | f0dcd6ab-9209-40a0-bed6-46f594657b4e |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | MYNXGRIP |
| Version Model Number | MX6721 |
| Catalog Number | MX6721 |
| Company DUNS | 132286761 |
| Company Name | Access Closure, Inc. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | true |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Operating and Storage Conditions
| Storage Environment Temperature | Between 0 and 25 Degrees Celsius |
| Storage Environment Temperature | Between 15 Degrees Celsius and 30 Degrees Celsius |
| Storage Environment Temperature | Between 15 Degrees Celsius and 30 Degrees Celsius |
| Storage Environment Temperature | Between 15 Degrees Celsius and 30 Degrees Celsius |
| Storage Environment Temperature | Between 15 Degrees Celsius and 30 Degrees Celsius |
| Storage Environment Temperature | Between 15 Degrees Celsius and 30 Degrees Celsius |
| Storage Environment Temperature | Between 15 Degrees Celsius and 30 Degrees Celsius |
| Storage Environment Temperature | Between 15 Degrees Celsius and 30 Degrees Celsius |
| Storage Environment Temperature | Between 15 Degrees Celsius and 30 Degrees Celsius |
| Storage Environment Temperature | Between 15 Degrees Celsius and 30 Degrees Celsius |
| Storage Environment Temperature | Between 15 Degrees Celsius and 30 Degrees Celsius |
| Storage Environment Temperature | Between 15 Degrees Celsius and 30 Degrees Celsius |
| Storage Environment Temperature | Between 15 Degrees Celsius and 30 Degrees Celsius |
| Storage Environment Temperature | Between 15 Degrees Celsius and 30 Degrees Celsius |
| Storage Environment Temperature | Between 15 Degrees Celsius and 30 Degrees Celsius |
| Storage Environment Temperature | Between 15 Degrees Celsius and 30 Degrees Celsius |
| Storage Environment Temperature | Between 15 Degrees Celsius and 30 Degrees Celsius |
| Storage Environment Temperature | Between 15 Degrees Celsius and 30 Degrees Celsius |
| Storage Environment Temperature | Between 15 Degrees Celsius and 30 Degrees Celsius |
| Storage Environment Temperature | Between 15 Degrees Celsius and 30 Degrees Celsius |
| Storage Environment Temperature | Between 15 Degrees Celsius and 30 Degrees Celsius |
| Storage Environment Temperature | Between 15 Degrees Celsius and 30 Degrees Celsius |
| Storage Environment Temperature | Between 15 Degrees Celsius and 30 Degrees Celsius |
| Storage Environment Temperature | Between 15 Degrees Celsius and 30 Degrees Celsius |
| Storage Environment Temperature | Between 15 Degrees Celsius and 30 Degrees Celsius |
| Storage Environment Temperature | Between 15 Degrees Celsius and 30 Degrees Celsius |
| Storage Environment Temperature | Between 15 Degrees Celsius and 30 Degrees Celsius |
| Storage Environment Temperature | Between 15 Degrees Celsius and 30 Degrees Celsius |
| Storage Environment Temperature | Between 15 Degrees Celsius and 30 Degrees Celsius |
| Storage Environment Temperature | Between 15 Degrees Celsius and 30 Degrees Celsius |
| Storage Environment Temperature | Between 15 Degrees Celsius and 30 Degrees Celsius |
| Storage Environment Temperature | Between 15 Degrees Celsius and 30 Degrees Celsius |
| Storage Environment Temperature | Between 15 Degrees Celsius and 30 Degrees Celsius |
| Storage Environment Temperature | Between 15 Degrees Celsius and 30 Degrees Celsius |
| Storage Environment Temperature | Between 15 Degrees Celsius and 30 Degrees Celsius |
| Storage Environment Temperature | Between 15 Degrees Celsius and 30 Degrees Celsius |
| Storage Environment Temperature | Between 15 Degrees Celsius and 30 Degrees Celsius |
| Storage Environment Temperature | Between 15 Degrees Celsius and 30 Degrees Celsius |
| Storage Environment Temperature | Between 15 Degrees Celsius and 30 Degrees Celsius |
| Storage Environment Temperature | Between 15 Degrees Celsius and 30 Degrees Celsius |
| Storage Environment Temperature | Between 15 Degrees Celsius and 30 Degrees Celsius |
| Storage Environment Temperature | Between 15 Degrees Celsius and 30 Degrees Celsius |
| Storage Environment Temperature | Between 15 Degrees Celsius and 30 Degrees Celsius |
| Storage Environment Temperature | Between 15 Degrees Celsius and 30 Degrees Celsius |
| Storage Environment Temperature | Between 15 Degrees Celsius and 30 Degrees Celsius |
| Storage Environment Temperature | Between 15 Degrees Celsius and 30 Degrees Celsius |
| Storage Environment Temperature | Between 15 Degrees Celsius and 30 Degrees Celsius |
| Storage Environment Temperature | Between 15 Degrees Celsius and 30 Degrees Celsius |
| Storage Environment Temperature | Between 15 Degrees Celsius and 30 Degrees Celsius |
| Storage Environment Temperature | Between 15 Degrees Celsius and 30 Degrees Celsius |
| Storage Environment Temperature | Between 15 Degrees Celsius and 30 Degrees Celsius |
| Storage Environment Temperature | Between 15 Degrees Celsius and 30 Degrees Celsius |
| Storage Environment Temperature | Between 15 Degrees Celsius and 30 Degrees Celsius |
| Storage Environment Temperature | Between 15 Degrees Celsius and 30 Degrees Celsius |
| Storage Environment Temperature | Between 15 Degrees Celsius and 30 Degrees Celsius |
| Storage Environment Temperature | Between 15 Degrees Celsius and 30 Degrees Celsius |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 10862028000410 [Primary] |
| GS1 | 20862028000417 [Package] Contains: 10862028000410 Package: BOX [10 Units] In Commercial Distribution |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Approval: P040044
FDA Product Code
| MGB | Device, hemostasis, vascular |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | true |
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 5 |
| Public Version Date | 2020-04-17 |
| Device Publish Date | 2017-08-23 |
On-Brand Devices [MYNXGRIP]
| 20862028000417 | MynxGrip 6F/7F |
| 20862028000400 | MynxGrip 5F |
Trademark Results [MYNXGRIP]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
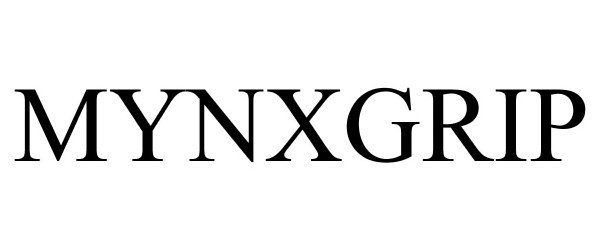 MYNXGRIP 85280686 4144053 Live/Registered |
AccessClosure, Inc. 2011-03-29 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.