Devon 31142113
GUDID 20884527016157
1530 Double Magnet Needle Counter,Black
Cardinal Health, Inc.
Needle holder, reusable| Primary Device ID | 20884527016157 |
| NIH Device Record Key | 1f0995d3-0053-4c8b-97d6-22305f8d9731 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | Devon |
| Version Model Number | 31142113 |
| Catalog Number | 31142113 |
| Company DUNS | 080935429 |
| Company Name | Cardinal Health, Inc. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | true |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | false |
| OTC Over-The-Counter | true |
Customer Support Contacts
| Phone | +1(508)261-8000 |
| covidien.udi@covidien.com | |
| Phone | +1(508)261-8000 |
| covidien.udi@covidien.com | |
| Phone | +1(508)261-8000 |
| covidien.udi@covidien.com | |
| Phone | +1(508)261-8000 |
| covidien.udi@covidien.com | |
| Phone | +1(508)261-8000 |
| covidien.udi@covidien.com | |
| Phone | +1(508)261-8000 |
| covidien.udi@covidien.com | |
| Phone | +1(508)261-8000 |
| covidien.udi@covidien.com | |
| Phone | +1(508)261-8000 |
| covidien.udi@covidien.com | |
| Phone | +1(508)261-8000 |
| covidien.udi@covidien.com | |
| Phone | +1(508)261-8000 |
| covidien.udi@covidien.com | |
| Phone | +1(508)261-8000 |
| covidien.udi@covidien.com | |
| Phone | +1(508)261-8000 |
| covidien.udi@covidien.com | |
| Phone | +1(508)261-8000 |
| covidien.udi@covidien.com | |
| Phone | +1(508)261-8000 |
| covidien.udi@covidien.com | |
| Phone | +1(508)261-8000 |
| covidien.udi@covidien.com | |
| Phone | +1(508)261-8000 |
| covidien.udi@covidien.com |
Operating and Storage Conditions
| Special Storage Condition, Specify | Between 0 and 0 *; |
| Special Storage Condition, Specify | Between 0 and 0 *; |
| Special Storage Condition, Specify | Between 0 and 0 *; |
| Special Storage Condition, Specify | Between 0 and 0 *; |
| Special Storage Condition, Specify | Between 0 and 0 *; |
| Special Storage Condition, Specify | Between 0 and 0 *; |
| Special Storage Condition, Specify | Between 0 and 0 *; |
| Special Storage Condition, Specify | Between 0 and 0 *; |
| Special Storage Condition, Specify | Between 0 and 0 *; |
| Special Storage Condition, Specify | Between 0 and 0 *; |
| Special Storage Condition, Specify | Between 0 and 0 *; |
| Special Storage Condition, Specify | Between 0 and 0 *; |
| Special Storage Condition, Specify | Between 0 and 0 *; |
| Special Storage Condition, Specify | Between 0 and 0 *; |
| Special Storage Condition, Specify | Between 0 and 0 *; |
| Special Storage Condition, Specify | Between 0 and 0 *; |
| Special Storage Condition, Specify | Between 0 and 0 *; |
| Special Storage Condition, Specify | Between 0 and 0 *; |
| Special Storage Condition, Specify | Between 0 and 0 *; |
| Special Storage Condition, Specify | Between 0 and 0 *; |
| Special Storage Condition, Specify | Between 0 and 0 *; |
| Special Storage Condition, Specify | Between 0 and 0 *; |
| Special Storage Condition, Specify | Between 0 and 0 *; |
| Special Storage Condition, Specify | Between 0 and 0 *; |
| Special Storage Condition, Specify | Between 0 and 0 *; |
| Special Storage Condition, Specify | Between 0 and 0 *; |
| Special Storage Condition, Specify | Between 0 and 0 *; |
| Special Storage Condition, Specify | Between 0 and 0 *; |
| Special Storage Condition, Specify | Between 0 and 0 *; |
| Special Storage Condition, Specify | Between 0 and 0 *; |
| Special Storage Condition, Specify | Between 0 and 0 *; |
| Special Storage Condition, Specify | Between 0 and 0 *; |
| Special Storage Condition, Specify | Between 0 and 0 *; |
| Special Storage Condition, Specify | Between 0 and 0 *; |
| Special Storage Condition, Specify | Between 0 and 0 *; |
| Special Storage Condition, Specify | Between 0 and 0 *; |
| Special Storage Condition, Specify | Between 0 and 0 *; |
| Special Storage Condition, Specify | Between 0 and 0 *; |
| Special Storage Condition, Specify | Between 0 and 0 *; |
| Special Storage Condition, Specify | Between 0 and 0 *; |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 10884527016150 [Package] Contains: 30884527016154 Package: PACK_OR_INNER_PACK [12 Units] In Commercial Distribution |
| GS1 | 20884527016157 [Package] Contains: 30884527016154 Package: CASE [96 Units] In Commercial Distribution |
| GS1 | 30884527016154 [Primary] |
FDA Product Code
| GAB | NEEDLE, SUTURING, DISPOSABLE |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | true |
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 2 |
| Public Version Date | 2019-02-15 |
| Device Publish Date | 2018-06-23 |
On-Brand Devices [Devon]
| 10884527020171 | Suture Removal Kit,7044 Suture Removal Kit |
| 20884527020154 | Suture Removal Kit,7042 Suture Removal Kit |
| 10884527020140 | Suture Removal Kit,7046 Suture Removal Kit |
| 20884527020062 | Suture Removal Kit,7044-MHH Suture Removal Kit |
| 20884527016546 | 1822 Foam Block Needle Counter |
| 20884527016539 | 1820 Foam Block Needle Counter |
| 10884527016525 | 1812 Foam Block Needle Counter |
| 20884527016508 | 1880 Foam Block Needle Counter |
| 20884527016492 | 1630 Foam Strip Needle Counter |
| 10884527016471 | 1810 Foam Block Needle Counter |
| 10884527016464 | 1100 Single Magnet Needle Counter,Black |
| 20884527016454 | 1842 Foam Block Needle Counter |
| 20884527016447 | 1910 Foam Block Needle Counter |
| 20884527016423 | 1920 Foam Block Needle Counter |
| 10884527016419 | 1930 Foam Block Needle Counter |
| 10884527016402 | 1922 Foam Block Needle Counter |
| 20884527016393 | 1960 Foam Block Needle Counter |
| 20884527016317 | 1840 Foam Block Needle Counter |
| 20884527016287 | 1330 Double Foam Strip Needle Counter |
| 10884527016273 | 1614 Foam Strip Needle Counter |
| 20884527016249 | 1540 Double Magnet Needle Counter,White |
| 10884527016235 | 1105 Foam Strip Needle Counter |
| 20884527016225 | 1360 Foam Strip Needle Counter |
| 10884527016211 | 1200 Double Magnet Needle Counter,Black |
| 10884527016204 | 1315 Foam Strip Needle Counter |
| 10884527016198 | 1314 Foam Strip Needle Counter |
| 10884527016181 | 1260 Double Magnet Needle Counter,Black |
| 20884527016171 | 1230 Double Magnet Needle Counter,Black |
| 10884527016167 | 1110 Single Magnet Needle Counter,Black |
| 20884527016157 | 1530 Double Magnet Needle Counter,Black |
| 20884527016041 | Skin Marker,Fine Tip |
| 10884527015962 | Printed Medication Labels |
| 20884527015952 | Utility Marker with Preprinted Labels |
| 10884527015917 | Utility Marker Labels |
| 10884527015900 | Anti-fog Solution,1910 Needle Counter |
| 10884527015894 | Scope Adjustment Bag |
| 20884527015884 | Anti-Fog Solution with Foam Pad |
| 20884527015839 | Tube Holder,Fixed Touch Fasten Strap |
| 10884527015825 | Tube Holder,Removable Touch Fasten Strap |
| 20884527015815 | Blade Shield Scalpel Holder |
| 10884527015801 | Instrument Pouch,Double Pocket |
| 20884527015792 | Micro Tip Wipe |
| 20884527011763 | Light Handle |
| 10884527011742 | Scope Adjustment Bag |
| 10884527011735 | Light Glove |
| 20884527011718 | Light Glove |
| 20884527011701 | Light Glove |
| 20884527011695 | Light Handle |
| 10884527011681 | Light Handle |
| 10884527011629 | 7075 Staple Remover |
Trademark Results [Devon]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
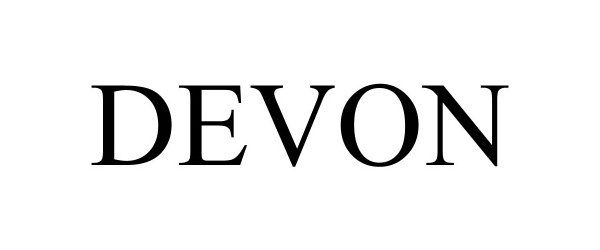 DEVON 98631766 not registered Live/Pending |
Cardinal Health 529, LLC 2024-07-03 |
 DEVON 98278891 not registered Live/Pending |
Hines Interests Limited Partnership 2023-11-20 |
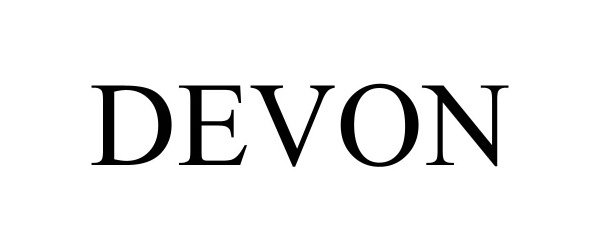 DEVON 98188439 not registered Live/Pending |
DEVON GUITARS 2023-09-20 |
 DEVON 97211388 not registered Live/Pending |
DEVCOR CAPITAL INC. 2022-01-10 |
 DEVON 88424843 not registered Live/Pending |
Cardinal Health 529, LLC 2019-05-10 |
 DEVON 85802470 4453636 Live/Registered |
ASCENT HEALTHCARE, LLC 2012-12-13 |
 DEVON 85382582 not registered Dead/Abandoned |
BLACK KNIGHT HOLDINGS, LLC 2011-07-27 |
 DEVON 81040117 1040117 Dead/Cancelled |
Advanco Inc. 0000-00-00 |
 DEVON 79063566 3712283 Dead/Cancelled |
CHERVON (CHINA) TRADING CO., LTD. 2008-08-07 |
 DEVON 78610143 3076726 Live/Registered |
HOULIHAN'S RESTAURANTS, INC. 2005-04-15 |
 DEVON 77859791 not registered Dead/Abandoned |
Devon Motorworks, LLC 2009-10-28 |
 DEVON 77759825 3736845 Live/Registered |
Devon Energy Corporation 2009-06-15 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.