Fischer Cone Biopsy Excisor
GUDID 20888937003776
Fischer Cone Biopsy Excisor; Small Wide Angle Extended
Coopersurgical, Inc.
Open-surgery electrosurgical electrode, monopolar, single-use| Primary Device ID | 20888937003776 |
| NIH Device Record Key | 9ee030ac-fec6-49cc-8e12-a348a9d5e2f3 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | Fischer Cone Biopsy Excisor |
| Version Model Number | 900-158 |
| Company DUNS | 801895244 |
| Company Name | Coopersurgical, Inc. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00888937003772 [Primary] |
| GS1 | 20888937003776 [Package] Contains: 00888937003772 Package: [5 Units] In Commercial Distribution |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K061651
FDA Product Code
| HGI | Electrocautery, Gynecologic (And Accessories) |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | true |
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 4 |
| Public Version Date | 2019-02-07 |
| Device Publish Date | 2016-08-16 |
On-Brand Devices [Fischer Cone Biopsy Excisor]
| 20888937003776 | Fischer Cone Biopsy Excisor; Small Wide Angle Extended |
| 20888937003769 | Fischer Cone Biopsy Excisor; Small Wide Angle |
| 20888937003745 | Fischer Cone Biopsy Excisor; Large Shallow |
| 20888937003738 | Fischer Cone Biopsy Excisor; Medium Extended |
| 20888937003721 | Fischer Cone Biopsy Excisor; Large |
| 20888937003714 | Fischer Cone Biopsy Excisor; Medium |
| 20888937003707 | Fischer Cone Biopsy Excisor; Small |
Trademark Results [Fischer Cone Biopsy Excisor]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
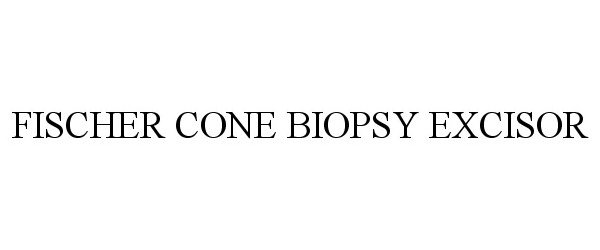 FISCHER CONE BIOPSY EXCISOR 85466592 4270743 Live/Registered |
CooperSurgical, Inc. 2011-11-07 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.