Cervex-Brush
GUDID 20888937014390
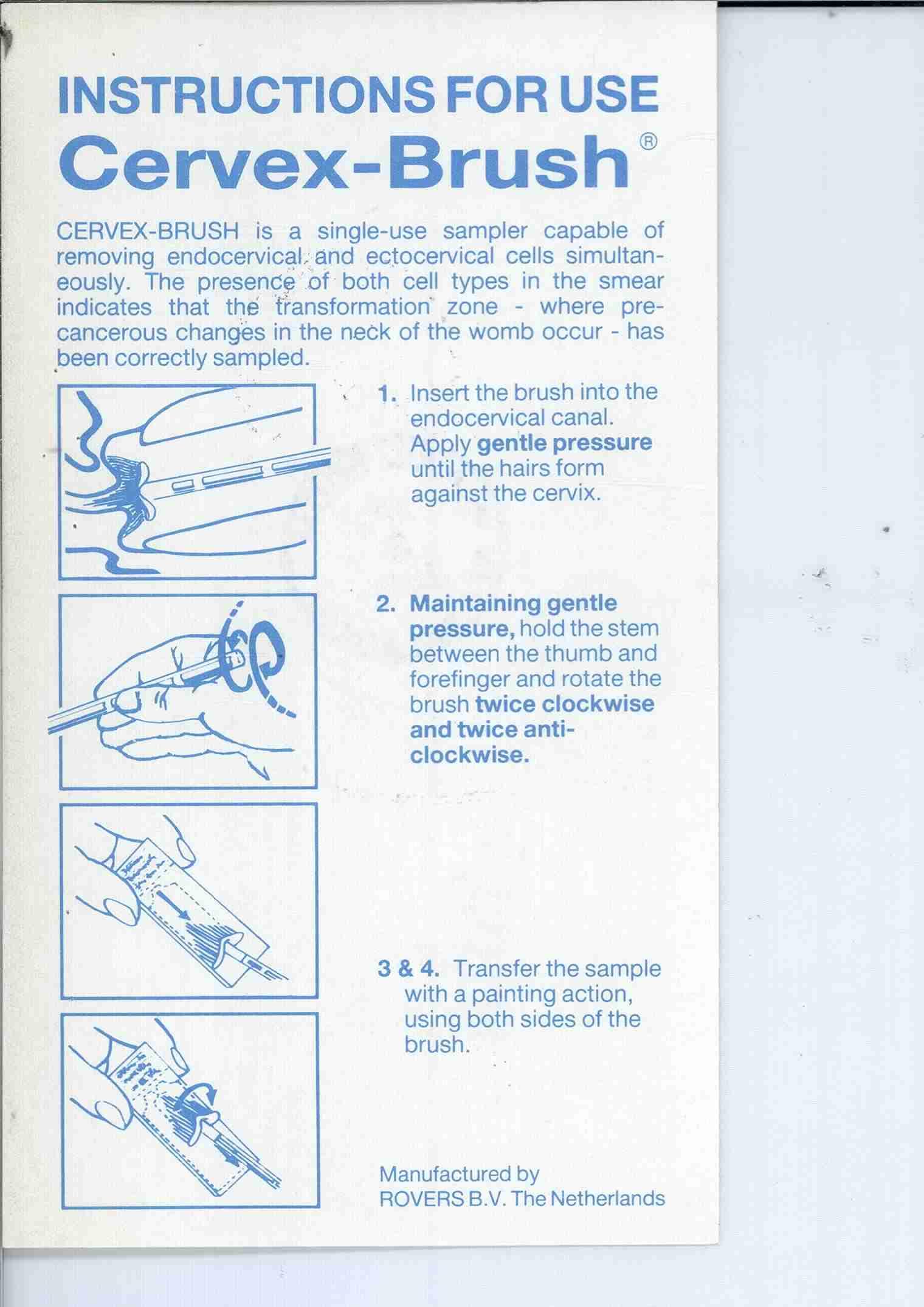
Cervex-Brush® Cervical Cell Sampler For simultaneous cytologic sampling of both the uterine ectocervix and endocervix for such subsequent evaluations
Coopersurgical, Inc.
Cervical cytology brush| Primary Device ID | 20888937014390 |
| NIH Device Record Key | adc5e921-40a5-4f77-b6b2-77e755544868 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | Cervex-Brush |
| Version Model Number | 8600 |
| Company DUNS | 801895244 |
| Company Name | Coopersurgical, Inc. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | true |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 20888937014390 [Primary] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K890848
FDA Product Code
| HHT | Spatula, Cervical, Cytological |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 3 |
| Public Version Date | 2018-07-06 |
| Device Publish Date | 2016-09-30 |
Devices Manufactured by Coopersurgical, Inc.
| 00888937028126 - Biopsy Medium | 2026-02-12 Biopsy Medium. 10 ml |
| 00888937028157 - Sperm Freezing Medium | 2026-02-12 Sperm Freezing Medium. 10ml |
| 00888937028218 - PVP Medium | 2026-02-12 PVP Medium with phenol red. 1 ml |
| 60888937028272 - Universal IVF Medium | 2026-02-12 Universal IVF Medium. 60 ml |
| 00888937028287 - Universal IVF Medium | 2026-02-12 Universal IVF Medium with phenol red. 60 ml |
| 00888937028317 - UTM | 2026-02-12 UTM Transfer Medium with phenol red. 10 ml |
| 00888937028324 - UTM | 2026-02-12 UTM Transfer Medium |
| 00888937028577 - Flushing Medium | 2026-02-12 Flushing Medium without Heparin. 125 ml |
Trademark Results [Cervex-Brush]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 CERVEX-BRUSH 74154444 1855648 Live/Registered |
ROVERS MEDICAL DEVICES B.V. 1991-04-05 |
 CERVEX-BRUSH 74075103 not registered Dead/Abandoned |
Unimar, Inc. 1990-07-03 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.