VERSABOND 71271440
GUDID 23596010507376
VERSABOND AB 40 GRAMS FORMULATION 2
Smith & Nephew, Inc.
Orthopaedic cement, non-medicated| Primary Device ID | 23596010507376 |
| NIH Device Record Key | 2c177609-8592-41f2-b581-d922708ff84d |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | VERSABOND |
| Version Model Number | 71271440 |
| Catalog Number | 71271440 |
| Company DUNS | 109903521 |
| Company Name | Smith & Nephew, Inc. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | true |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
| Phone | +1(800)821-5700 |
| gudid@smith-nephew.com | |
| Phone | +1(800)821-5700 |
| gudid@smith-nephew.com | |
| Phone | +1(800)821-5700 |
| gudid@smith-nephew.com | |
| Phone | +1(800)821-5700 |
| gudid@smith-nephew.com | |
| Phone | +1(800)821-5700 |
| gudid@smith-nephew.com | |
| Phone | +1(800)821-5700 |
| gudid@smith-nephew.com | |
| Phone | +1(800)821-5700 |
| gudid@smith-nephew.com | |
| Phone | +1(800)821-5700 |
| gudid@smith-nephew.com | |
| Phone | +1(800)821-5700 |
| gudid@smith-nephew.com | |
| Phone | +1(800)821-5700 |
| gudid@smith-nephew.com | |
| Phone | +1(800)821-5700 |
| gudid@smith-nephew.com | |
| Phone | +1(800)821-5700 |
| gudid@smith-nephew.com | |
| Phone | +1(800)821-5700 |
| gudid@smith-nephew.com | |
| Phone | +1(800)821-5700 |
| gudid@smith-nephew.com |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 03596010507372 [Primary] |
| GS1 | 23596010507376 [Package] Contains: 03596010507372 Package: BX [10 Units] In Commercial Distribution |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K022688
FDA Product Code
| LOD | BONE CEMENT |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | true |
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 4 |
| Public Version Date | 2020-01-31 |
| Device Publish Date | 2015-09-28 |
On-Brand Devices [VERSABOND]
| 23596010507376 | VERSABOND AB 40 GRAMS FORMULATION 2 |
| 23596010494720 | VERSABOND 40 GRAMS FORMULATION 2 |
| 23596010411024 | VERSABOND 40 GRAMS |
Trademark Results [VERSABOND]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
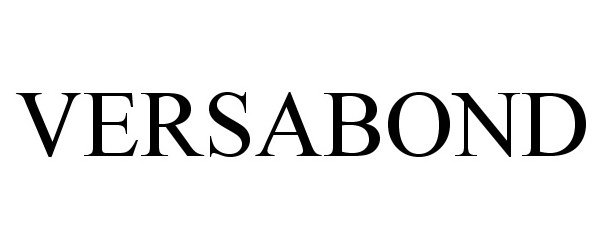 VERSABOND 86963890 5266963 Live/Registered |
SWIMC LLC 2016-04-04 |
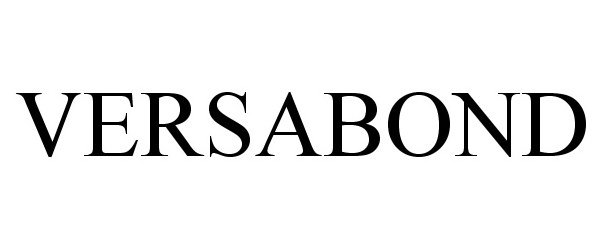 VERSABOND 86435118 4802572 Live/Registered |
PPG INDUSTRIES OHIO, INC. 2014-10-27 |
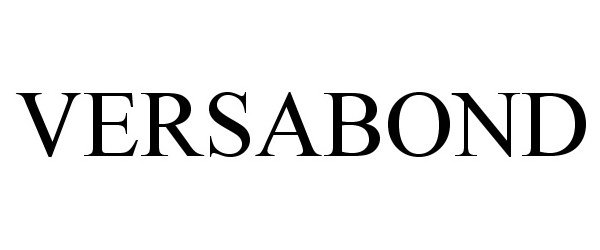 VERSABOND 86056420 not registered Dead/Abandoned |
M-D Building Products, Inc. 2013-09-05 |
 VERSABOND 75574557 2502446 Live/Registered |
SMITH & NEPHEW, INC. 1998-10-22 |
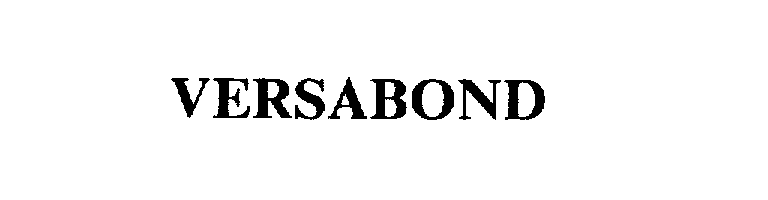 VERSABOND 75474898 2287412 Live/Registered |
CUSTOM BUILDING PRODUCTS, LLC 1998-04-27 |
 VERSABOND 74044785 1763674 Dead/Cancelled |
XEROX CORPORATION 1990-04-02 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.