PILLING 355832
GUDID 24026704523065
MARKING RING 10MM 10/PK
TELEFLEX INCORPORATED
Dental material cartridge-plunging applicator, two-component, pneumatic| Primary Device ID | 24026704523065 |
| NIH Device Record Key | 5ff9298f-2bcc-467b-ace9-cc97a9bdf8a7 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | PILLING |
| Version Model Number | IPN006044 |
| Catalog Number | 355832 |
| Company DUNS | 002348191 |
| Company Name | TELEFLEX INCORPORATED |
| Device Count | 1 |
| DM Exempt | true |
| Pre-market Exempt | true |
| MRI Safety Status | MR Unsafe |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | true |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
| Phone | +1(919)544-8000 |
| CS@TELEFLEX.COM | |
| Phone | +1(919)544-8000 |
| CS@TELEFLEX.COM | |
| Phone | +1(919)544-8000 |
| CS@TELEFLEX.COM | |
| Phone | +1(919)544-8000 |
| CS@TELEFLEX.COM | |
| Phone | +1(919)544-8000 |
| CS@TELEFLEX.COM | |
| Phone | +1(919)544-8000 |
| CS@TELEFLEX.COM | |
| Phone | +1(919)544-8000 |
| CS@TELEFLEX.COM | |
| Phone | +1(919)544-8000 |
| CS@TELEFLEX.COM | |
| Phone | +1(919)544-8000 |
| CS@TELEFLEX.COM | |
| Phone | +1(919)544-8000 |
| CS@TELEFLEX.COM | |
| Phone | +1(919)544-8000 |
| CS@TELEFLEX.COM | |
| Phone | +1(919)544-8000 |
| CS@TELEFLEX.COM | |
| Phone | +1(919)544-8000 |
| CS@TELEFLEX.COM | |
| Phone | +1(919)544-8000 |
| CS@TELEFLEX.COM | |
| Phone | +1(919)544-8000 |
| CS@TELEFLEX.COM | |
| Phone | +1(919)544-8000 |
| CS@TELEFLEX.COM | |
| Phone | +1(919)544-8000 |
| CS@TELEFLEX.COM | |
| Phone | +1(919)544-8000 |
| CS@TELEFLEX.COM | |
| Phone | +1(919)544-8000 |
| CS@TELEFLEX.COM | |
| Phone | +1(919)544-8000 |
| CS@TELEFLEX.COM | |
| Phone | +1(919)544-8000 |
| CS@TELEFLEX.COM | |
| Phone | +1(919)544-8000 |
| CS@TELEFLEX.COM | |
| Phone | +1(919)544-8000 |
| CS@TELEFLEX.COM | |
| Phone | +1(919)544-8000 |
| CS@TELEFLEX.COM | |
| Phone | +1(919)544-8000 |
| CS@TELEFLEX.COM | |
| Phone | +1(919)544-8000 |
| CS@TELEFLEX.COM | |
| Phone | +1(919)544-8000 |
| CS@TELEFLEX.COM | |
| Phone | +1(919)544-8000 |
| CS@TELEFLEX.COM | |
| Phone | +1(919)544-8000 |
| CS@TELEFLEX.COM | |
| Phone | +1(919)544-8000 |
| CS@TELEFLEX.COM | |
| Phone | +1(919)544-8000 |
| CS@TELEFLEX.COM | |
| Phone | +1(919)544-8000 |
| CS@TELEFLEX.COM |
Device Dimensions
| Width | 10 Millimeter |
| Width | 10 Millimeter |
| Width | 10 Millimeter |
| Width | 10 Millimeter |
| Width | 10 Millimeter |
| Width | 10 Millimeter |
| Width | 10 Millimeter |
| Width | 10 Millimeter |
| Width | 10 Millimeter |
| Width | 10 Millimeter |
| Width | 10 Millimeter |
| Width | 10 Millimeter |
| Width | 10 Millimeter |
| Width | 10 Millimeter |
| Width | 10 Millimeter |
| Width | 10 Millimeter |
| Width | 10 Millimeter |
| Width | 10 Millimeter |
| Width | 10 Millimeter |
| Width | 10 Millimeter |
| Width | 10 Millimeter |
| Width | 10 Millimeter |
| Width | 10 Millimeter |
| Width | 10 Millimeter |
| Width | 10 Millimeter |
| Width | 10 Millimeter |
| Width | 10 Millimeter |
| Width | 10 Millimeter |
| Width | 10 Millimeter |
| Width | 10 Millimeter |
| Width | 10 Millimeter |
| Width | 10 Millimeter |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 04026704523061 [Primary] |
| GS1 | 24026704523065 [Package] Contains: 04026704523061 Package: Bag [10 Units] In Commercial Distribution |
FDA Product Code
| MAB | Marker, cardiopulmonary bypass (vein marker) |
Sterilization
| Steralize Prior To Use | true |
| Device Is Sterile | false |
[24026704523065]
Moist Heat or Steam Sterilization
[24026704523065]
Moist Heat or Steam Sterilization
[24026704523065]
Moist Heat or Steam Sterilization
[24026704523065]
Moist Heat or Steam Sterilization
[24026704523065]
Moist Heat or Steam Sterilization
[24026704523065]
Moist Heat or Steam Sterilization
[24026704523065]
Moist Heat or Steam Sterilization
[24026704523065]
Moist Heat or Steam Sterilization
[24026704523065]
Moist Heat or Steam Sterilization
[24026704523065]
Moist Heat or Steam Sterilization
[24026704523065]
Moist Heat or Steam Sterilization
[24026704523065]
Moist Heat or Steam Sterilization
[24026704523065]
Moist Heat or Steam Sterilization
[24026704523065]
Moist Heat or Steam Sterilization
[24026704523065]
Moist Heat or Steam Sterilization
[24026704523065]
Moist Heat or Steam Sterilization
[24026704523065]
Moist Heat or Steam Sterilization
[24026704523065]
Moist Heat or Steam Sterilization
[24026704523065]
Moist Heat or Steam Sterilization
[24026704523065]
Moist Heat or Steam Sterilization
[24026704523065]
Moist Heat or Steam Sterilization
[24026704523065]
Moist Heat or Steam Sterilization
[24026704523065]
Moist Heat or Steam Sterilization
[24026704523065]
Moist Heat or Steam Sterilization
[24026704523065]
Moist Heat or Steam Sterilization
[24026704523065]
Moist Heat or Steam Sterilization
[24026704523065]
Moist Heat or Steam Sterilization
[24026704523065]
Moist Heat or Steam Sterilization
[24026704523065]
Moist Heat or Steam Sterilization
[24026704523065]
Moist Heat or Steam Sterilization
[24026704523065]
Moist Heat or Steam Sterilization
[24026704523065]
Moist Heat or Steam Sterilization
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 3 |
| Public Version Date | 2021-11-17 |
| Device Publish Date | 2016-09-16 |
On-Brand Devices [PILLING]
| 24026704555554 | JAKO-CHERRY LARYNGOSCOPE TYPE 2 NEWBORN |
| 04026704535750 | HOLINGER ESOPHAGOSCOPE 7X30 |
| 04026704535606 | HOLINGER BRONCHOSCOPE 5X40 |
| 34026704630371 | PUNCH MDP-44K NON-STERILE 4.4 MILLIMETERS |
| 34026704630364 | PUNCH MDP-44K NON-STERILE 4.4 MILLIMETERS |
| 34026704630357 | PUNCH DP-56K NON-STERILE 5.6 MILLIMETERS |
| 34026704630340 | PUNCH DP-52K NON-STERILE 5.2 MILLIMETERS |
| 34026704630333 | PUNCH DP-48 NON-STERILE 4.8 MILLIMETERS |
| 34026704630326 | PUNCH DP-44K NON-STERILE 4.4 MILLIMETERS |
| 34026704630319 | PUNCH DP-40 NON-STERILE 4.0 MILLIMETERS |
| 14026704534903 | CLJ LARYNGECTOMY TUBE #12 |
| 14026704534897 | CLJ LARYNGECTOMY TUBE #11 |
| 14026704534880 | CLJ LARYNGECTOMY TUBE #10 |
| 14026704534873 | CLJ LARYNGECTOMY TUBE #9 |
| 14026704534866 | CLJ LARYNGECTOMY TUBE #8 |
| 14026704534859 | CLJ LARYNGECTOMY TUBE #7 |
| 14026704534842 | CLJ LARYNGECTOMY TUBE #6 |
| 14026704534835 | CLJ TRACHEA TUBE #9 EXTRA LONG |
| 14026704534828 | CLJ TRACHEA TUBE #8 EXTRA LONG |
| 14026704534811 | CLJ TRACHEA TUBE #7 EXTRA LONG |
| 14026704534804 | CLJ TRACHEA TUBE #6 EXTRA LONG |
| 14026704534798 | CLJ TRACHEA TUBE #5 EXTRA LONG |
| 14026704534781 | CJ TRACHEA TUBE #6 SHORT W/15 MILLIMETER ADAPTOR |
| 14026704534774 | CJ TRACHEA TUBE #4 SHORT W/15 MILLIMETER ADAPTOR |
| 14026704534767 | CLJ TRACHEA TUBE #8 SHORT W/15 MILLIMETER ADAPTOR |
| 14026704534750 | CLJ TRACHEA TUBE #7 SHORT W/15 MILLIMETER ADAPTOR |
| 14026704534743 | CLJ TRACHEA TUBE #6 SHORT W/15 MILLIMETER ADAPTOR |
| 14026704534736 | CLJ TRACHEA TUBE #5 SHORT W/15 MILLIMETER ADAPTOR |
| 14026704534729 | CLJ TRACHEA TUBE #4 SHORT W/15 MILLIMETER ADAPTOR |
| 14026704534712 | CJ TRACHEA TUBE ORIGINAL #10 W/15 MILLIMETER ADAPT |
| 14026704534705 | CJ TRACHEA TUBE ORIGINAL #8 W/15 MILLIMETER ADAPTO |
| 14026704534699 | CJ TRACHEA TUBE ORIGINAL #7 W/15 MILLIMETER ADAPTO |
| 14026704534682 | CJ TRACHEA TUBE ORIGINAL #6 W/15 MILLIMETER ADAPTO |
| 14026704534675 | CJ TRACHEA TUBE ORIGINAL #5 W/15 MILLIMETER ADAPTO |
| 14026704534668 | CJ TRACHEA TUBE ORIGINAL #4 W/15 MILLIMETER ADAPTO |
| 14026704534651 | CJ TRACHEA TUBE ORIGINAL #3 W/15 MILLIMETER ADAPTO |
| 14026704534644 | CLJ TRACHEA TUBE IMPROVED #10 W/15 MILLIMETER ADAP |
| 14026704534637 | CLJ TRACHEA TUBE IMPROVED #8 W/15 MILLIMETER ADAPT |
| 14026704534620 | CLJ TRACHEA TUBE IMPROVED #7 W/15 MILLIMETER ADAPT |
| 14026704534613 | CLJ TRACHEA TUBE IMPROVED #6 W/15 MILLIMETER ADAPT |
| 14026704534606 | CLJ TRACHEA TUBE IMPROVED #5 W/15 MILLIMETER ADAPT |
| 14026704534590 | CLJ TRACHEA TUBE IMPROVED #4 W/15 MILLIMETER ADAPT |
| 14026704534583 | CJ TRACHEA TUBE #8 SHORT STAINLESS STEEL |
| 14026704534576 | CJ TRACHEA TUBE #7 SHORT STAINLESS STEEL |
| 14026704534569 | CJ TRACHEA TUBE #6 SHORT STAINLESS STEEL |
| 14026704534552 | CJ TRACHEA TUBE #5 SHORT STAINLESS STEEL |
| 14026704534545 | CJ TRACHEA TUBE #4 SHORT STAINLESS STEEL |
| 14026704534538 | CJ TRACHEA TUBE #3 SHORT STAINLESS STEEL |
| 14026704534521 | TRACHEA TUBE CLJ IMPROVED #10 SHORT STAINLESS STEE |
| 14026704534514 | CLJ TRACHEA TUBE #9 SHORT STAINLESS STEEL |
Trademark Results [PILLING]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
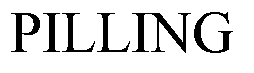 PILLING 71329492 0299162 Live/Registered |
PILLING CO. 1932-08-12 |
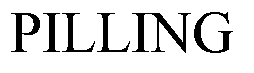 PILLING 71329492 0299162 Live/Registered |
GEORGE P. PILLING & SON CO. 1932-08-12 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.