Apex
GUDID 30076855700045
oral syringe
COMPASS HEALTH BRANDS CORP.
Irrigation syringe| Primary Device ID | 30076855700045 |
| NIH Device Record Key | 1a3e5cec-1960-4e58-959a-4bd931e87ae0 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | Apex |
| Version Model Number | 70004 |
| Company DUNS | 827221698 |
| Company Name | COMPASS HEALTH BRANDS CORP. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | true |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | false |
| OTC Over-The-Counter | false |
Customer Support Contacts
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00076855700044 [Primary] |
| GS1 | 30076855700045 [Package] Contains: 00076855700044 Package: [144 Units] In Commercial Distribution |
FDA Product Code
| KYW | Container, Liquid Medication, Graduated |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 2 |
| Public Version Date | 2023-09-01 |
| Device Publish Date | 2023-01-08 |
On-Brand Devices [Apex]
| 40076855710218 | DELUXE CONTACT EYE CASE CARDED |
| 00076855710166 | LENS-MATE CONTACT LENS CASES |
| 30076855702568 | 70256 |
| 50076855701350 | EAR THERMOMETER (1 SEC) |
| 40076855700615 | DIGITAL THERM W/FLEX TIP BOXED |
| 30076855700564 | DIGITAL THERM COLOR - CLAMSHELLED |
| 40076855700400 | DIGITAL PACIFIER THERMOMETER |
| 50076855700339 | DIGITAL THERM BOXED |
| 40076855700325 | TEMP*QWIK THERMOMETER CARDED |
| 30076855700311 | PEDIATRIC KIT (INC. CLASS 1 AND 2) |
| 30076855700717 | 70071 |
| 30076855700298 | 70029 |
| 00076855700280 | 70028 |
| 30076855700687 | ULTRA PILL SPLITTER CARDED |
| 30076855700588 | DELUXE PILL SPLITTER CARDED |
| 30854875008040 | 70256FSA |
| 30041520876479 | MEDICINE SPOON/SUPER DROPPER BLISTERED QUALITY CHOICE |
| 30635515992642 | Super Dropper Assorted Quality Choice |
| 30076855700793 | DUAL POCKET MED PACK CARDED W/ APEX/CAREX EMBOSSED |
| 30076855700755 | POCKET MED PACK W/TRAY 7 DAYS LG. AM/PM PRINT 7-DAYS OF WEEK W/ APEX-CAREX EMBOSSED |
| 30076855100050 | Super Dropper HOT STAMPED CELLO WRAP |
| 00041520865786 | MEDI PLANNER DELUXE BOXED AHOLD |
| 30076855700274 | MEDI TRAY w/ TRANS. BLUE DAYPLANNER CELLOED - NO LABEL |
| 00041520865793 | XXL PILL ORG BOXED AHOLD ASSORTED #86579 |
| 30635515992673 | DUAL POCKET MED PACK CARDED W/ AMPM QUALITY CHOICE |
| 30076855700854 | XL Bubble Lok Assorted Celloed |
| 30635515992727 | XL Bubble Lok Assorted Quality Choice |
| 30635515992703 | Twice-A-Day Pill Organizer |
| 30041520865770 | Twice-A-Day Pill Organizer |
| 30041520865749 | Ultra Pill Organizer Carded Ahold |
| 30076855700779 | POCKET MED PAK ASSORTED IMPRINTED CELLO WRAPPED GIANT PHARMACY |
| 30076855005089 | Eye / Ear Dropper |
| 30076855000312 | Soft Foam Ear Plugs - 6 Pair |
| 30076855000305 | Soft Foam Ear Plugs - 4 Pair |
| 30076855000275 | Air Pocket Ear Plugs |
| 30076855000268 | Flex-Tone Ear Plugs |
| 30076855000206 | Foam Ear Plugs with Cord |
| 30076855000145 | Silicone Ear Plugs (Small) - 6 Pair |
| 30076855000107 | Silicone Ear Plugs - 2 Pair |
| 30076855700052 | Super Dropper |
| 30076855700045 | oral syringe |
| 30076855700014 | MEDICINE SPOON |
| 30076855619194 | Pocket/Purse Pill Box - 2 Pack |
| 30076855605050 | Super Dropper - 2 Pack |
| 30076855601052 | MEDICINE SPOON/SUPER DROPPER BLISTERED |
| 00076855700723 | ULTRA PILL OGANIZER CARDED |
| 30076855700595 | Weekly Twice-A-Day Pill Organizer |
| 30076855700540 | Pill Tote™ |
| 30076855700533 | MediPlanner® Deluxe / II |
| 30076855700472 | 7-Day AM/PM XXL Pill Organizer |
Trademark Results [Apex]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 APEX 98811987 not registered Live/Pending |
AFTERMARKET PERFORMANCE EXPRESS 2024-10-21 |
 APEX 98805060 not registered Live/Pending |
Dongguan Luxshare Technology Co., Ltd. 2024-10-16 |
 APEX 98733536 not registered Live/Pending |
Apex natural nutrition inc. 2024-09-04 |
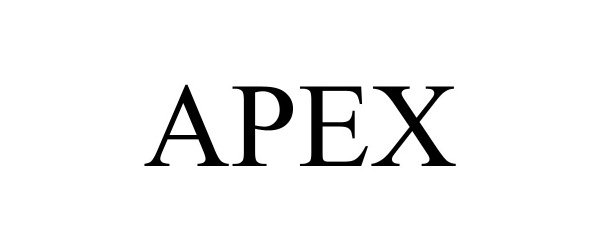 APEX 98674096 not registered Live/Pending |
Medtronic Xomed, Inc. 2024-07-30 |
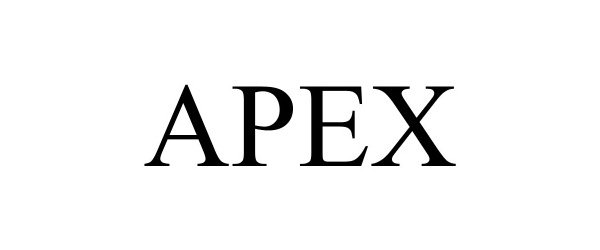 APEX 98673954 not registered Live/Pending |
Covert Instruments LLC 2024-07-30 |
 APEX 98668271 not registered Live/Pending |
Smith-Root, Inc. 2024-07-26 |
 APEX 98662611 not registered Live/Pending |
FRC Global Inc. 2024-07-23 |
 APEX 98644654 not registered Live/Pending |
Blume, Phillip James 2024-07-11 |
 APEX 98636442 not registered Live/Pending |
Catalina Carpet Mills Inc. 2024-07-08 |
 APEX 98616917 not registered Live/Pending |
Lander Hospitality Holdings LLC 2024-06-25 |
 APEX 98616917 not registered Live/Pending |
Hines, Victor Nunzio 2024-06-25 |
 APEX 98616917 not registered Live/Pending |
Comes, Bryan 2024-06-25 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.