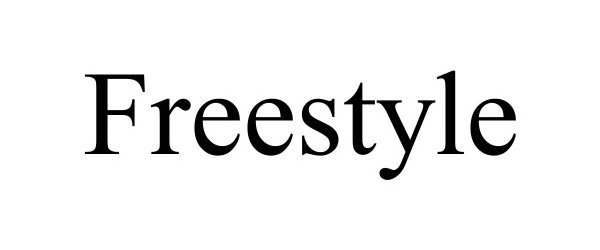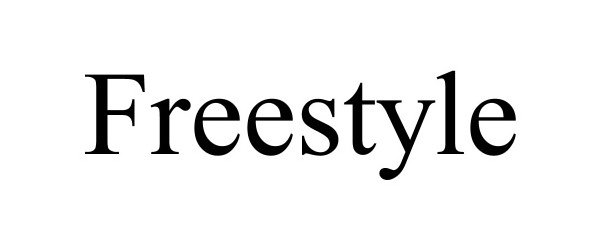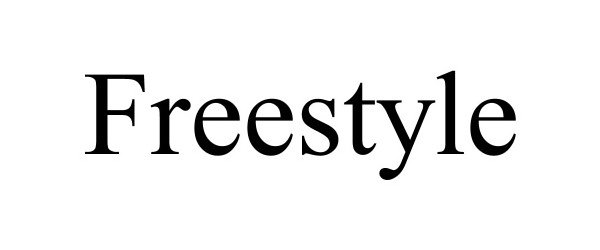FreeStyle 70428
GUDID 30093815704288
FreeStyle Unistik 2
ABBOTT DIABETES CARE INC
Glucose monitoring system IVD, home-use| Primary Device ID | 30093815704288 |
| NIH Device Record Key | de5ac188-4a67-46b4-8b3c-763fa36b5abe |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | FreeStyle |
| Version Model Number | 70428 |
| Catalog Number | 70428 |
| Company DUNS | 966390890 |
| Company Name | ABBOTT DIABETES CARE INC |
| Device Count | 200 |
| DM Exempt | false |
| Pre-market Exempt | true |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | true |
| Manufacturing Date | true |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | false |
| OTC Over-The-Counter | false |
Customer Support Contacts
| Phone | +1(888)522-5226 |
| xx@xx.xx |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00093815704287 [Primary] |
| GS1 | 10093815704284 [Unit of Use] |
| GS1 | 30093815704288 [Package] Contains: 00093815704287 Package: Case [30 Units] In Commercial Distribution |
| NDC/NHRIC | 99073-0704-28 [Secondary] |
FDA Product Code
| FMK | Lancet, blood |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | true |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2018-08-03 |
| Device Publish Date | 2018-07-03 |
On-Brand Devices [FreeStyle]
| 30357599000661 | FreeStyle Lancing Device II |
| 30699073707926 | FreeStyle Blood Glucose Test Strips |
| 20699073704324 | FreeStyle Control Solution |
| 20699073140023 | FreeStyle Control Solution |
| 30699073130014 | FreeStyle Lancets 28 Gauge |
| 30699073124501 | FreeStyle Blood Glucose Test Strips |
| 30699073124013 | FreeStyle Blood Glucose Test Strips |
| 40699073121019 | FreeStyle Blood Glucose Test Strips |
| 40699073120500 | FreeStyle Blood Glucose Test Strips |
| 30093815704288 | FreeStyle Unistik 2 |
Trademark Results [FreeStyle]
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.