ORACOTE® Absorbable Collagen Wound Dressing for Dental Surgery #ORACOTE-PKG-10
GUDID 30381780000314
SALVIN OraCOTE® Absorbable Collagen Wound Dressing for Dental Surgery, 3/4in x 1 1/2in
Integra Lifesciences Corporation
Collagen wound matrix dressing| Primary Device ID | 30381780000314 |
| NIH Device Record Key | f0393dc7-8f04-4c72-8b5a-1b3507885be2 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | ORACOTE® Absorbable Collagen Wound Dressing for Dental Surgery |
| Version Model Number | #ORACOTE-PKG-10 |
| Catalog Number | #ORACOTE-PKG-10 |
| Company DUNS | 083171244 |
| Company Name | Integra Lifesciences Corporation |
| Device Count | 10 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
| Phone | +1(800)654-2873 |
| custsvcnj@integralife.com | |
| Phone | +1(800)654-2873 |
| custsvcnj@integralife.com | |
| Phone | +1(800)654-2873 |
| custsvcnj@integralife.com | |
| Phone | +1(800)654-2873 |
| custsvcnj@integralife.com | |
| Phone | +1(800)654-2873 |
| custsvcnj@integralife.com | |
| Phone | +1(800)654-2873 |
| custsvcnj@integralife.com | |
| Phone | +1(800)654-2873 |
| custsvcnj@integralife.com | |
| Phone | +1(800)654-2873 |
| custsvcnj@integralife.com | |
| Phone | +1(800)654-2873 |
| custsvcnj@integralife.com | |
| Phone | +1(800)654-2873 |
| custsvcnj@integralife.com | |
| Phone | +1(800)654-2873 |
| custsvcnj@integralife.com | |
| Phone | +1(800)654-2873 |
| custsvcnj@integralife.com | |
| Phone | +1(800)654-2873 |
| custsvcnj@integralife.com | |
| Phone | +1(800)654-2873 |
| custsvcnj@integralife.com | |
| Phone | +1(800)654-2873 |
| custsvcnj@integralife.com | |
| Phone | +1(800)654-2873 |
| custsvcnj@integralife.com | |
| Phone | +1(800)654-2873 |
| custsvcnj@integralife.com | |
| Phone | +1(800)654-2873 |
| custsvcnj@integralife.com | |
| Phone | +1(800)654-2873 |
| custsvcnj@integralife.com | |
| Phone | +1(800)654-2873 |
| custsvcnj@integralife.com | |
| Phone | +1(800)654-2873 |
| custsvcnj@integralife.com | |
| Phone | +1(800)654-2873 |
| custsvcnj@integralife.com | |
| Phone | +1(800)654-2873 |
| custsvcnj@integralife.com | |
| Phone | +1(800)654-2873 |
| custsvcnj@integralife.com | |
| Phone | +1(800)654-2873 |
| custsvcnj@integralife.com | |
| Phone | +1(800)654-2873 |
| custsvcnj@integralife.com | |
| Phone | +1(800)654-2873 |
| custsvcnj@integralife.com | |
| Phone | +1(800)654-2873 |
| custsvcnj@integralife.com | |
| Phone | +1(800)654-2873 |
| custsvcnj@integralife.com |
Device Dimensions
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
Operating and Storage Conditions
| Special Storage Condition, Specify | Between 0 and 0 *Avoid excessive heat and humidity |
| Special Storage Condition, Specify | Between 0 and 0 *Avoid excessive heat and humidity |
| Special Storage Condition, Specify | Between 0 and 0 *Avoid excessive heat and humidity |
| Special Storage Condition, Specify | Between 0 and 0 *Avoid excessive heat and humidity |
| Special Storage Condition, Specify | Between 0 and 0 *Avoid excessive heat and humidity |
| Special Storage Condition, Specify | Between 0 and 0 *Avoid excessive heat and humidity |
| Special Storage Condition, Specify | Between 0 and 0 *Avoid excessive heat and humidity |
| Special Storage Condition, Specify | Between 0 and 0 *Avoid excessive heat and humidity |
| Special Storage Condition, Specify | Between 0 and 0 *Avoid excessive heat and humidity |
| Special Storage Condition, Specify | Between 0 and 0 *Avoid excessive heat and humidity |
| Special Storage Condition, Specify | Between 0 and 0 *Avoid excessive heat and humidity |
| Special Storage Condition, Specify | Between 0 and 0 *Avoid excessive heat and humidity |
| Special Storage Condition, Specify | Between 0 and 0 *Avoid excessive heat and humidity |
| Special Storage Condition, Specify | Between 0 and 0 *Avoid excessive heat and humidity |
| Special Storage Condition, Specify | Between 0 and 0 *Avoid excessive heat and humidity |
| Special Storage Condition, Specify | Between 0 and 0 *Avoid excessive heat and humidity |
| Special Storage Condition, Specify | Between 0 and 0 *Avoid excessive heat and humidity |
| Special Storage Condition, Specify | Between 0 and 0 *Avoid excessive heat and humidity |
| Special Storage Condition, Specify | Between 0 and 0 *Avoid excessive heat and humidity |
| Special Storage Condition, Specify | Between 0 and 0 *Avoid excessive heat and humidity |
| Special Storage Condition, Specify | Between 0 and 0 *Avoid excessive heat and humidity |
| Special Storage Condition, Specify | Between 0 and 0 *Avoid excessive heat and humidity |
| Special Storage Condition, Specify | Between 0 and 0 *Avoid excessive heat and humidity |
| Special Storage Condition, Specify | Between 0 and 0 *Avoid excessive heat and humidity |
| Special Storage Condition, Specify | Between 0 and 0 *Avoid excessive heat and humidity |
| Special Storage Condition, Specify | Between 0 and 0 *Avoid excessive heat and humidity |
| Special Storage Condition, Specify | Between 0 and 0 *Avoid excessive heat and humidity |
| Special Storage Condition, Specify | Between 0 and 0 *Avoid excessive heat and humidity |
| Special Storage Condition, Specify | Between 0 and 0 *Avoid excessive heat and humidity |
| Special Storage Condition, Specify | Between 0 and 0 *Avoid excessive heat and humidity |
| Special Storage Condition, Specify | Between 0 and 0 *Avoid excessive heat and humidity |
| Special Storage Condition, Specify | Between 0 and 0 *Avoid excessive heat and humidity |
| Special Storage Condition, Specify | Between 0 and 0 *Avoid excessive heat and humidity |
| Special Storage Condition, Specify | Between 0 and 0 *Avoid excessive heat and humidity |
| Special Storage Condition, Specify | Between 0 and 0 *Avoid excessive heat and humidity |
| Special Storage Condition, Specify | Between 0 and 0 *Avoid excessive heat and humidity |
| Special Storage Condition, Specify | Between 0 and 0 *Avoid excessive heat and humidity |
| Special Storage Condition, Specify | Between 0 and 0 *Avoid excessive heat and humidity |
| Special Storage Condition, Specify | Between 0 and 0 *Avoid excessive heat and humidity |
| Special Storage Condition, Specify | Between 0 and 0 *Avoid excessive heat and humidity |
| Special Storage Condition, Specify | Between 0 and 0 *Avoid excessive heat and humidity |
| Special Storage Condition, Specify | Between 0 and 0 *Avoid excessive heat and humidity |
| Special Storage Condition, Specify | Between 0 and 0 *Avoid excessive heat and humidity |
| Special Storage Condition, Specify | Between 0 and 0 *Avoid excessive heat and humidity |
| Special Storage Condition, Specify | Between 0 and 0 *Avoid excessive heat and humidity |
| Special Storage Condition, Specify | Between 0 and 0 *Avoid excessive heat and humidity |
| Special Storage Condition, Specify | Between 0 and 0 *Avoid excessive heat and humidity |
| Special Storage Condition, Specify | Between 0 and 0 *Avoid excessive heat and humidity |
| Special Storage Condition, Specify | Between 0 and 0 *Avoid excessive heat and humidity |
| Special Storage Condition, Specify | Between 0 and 0 *Avoid excessive heat and humidity |
| Special Storage Condition, Specify | Between 0 and 0 *Avoid excessive heat and humidity |
| Special Storage Condition, Specify | Between 0 and 0 *Avoid excessive heat and humidity |
| Special Storage Condition, Specify | Between 0 and 0 *Avoid excessive heat and humidity |
| Special Storage Condition, Specify | Between 0 and 0 *Avoid excessive heat and humidity |
| Special Storage Condition, Specify | Between 0 and 0 *Avoid excessive heat and humidity |
| Special Storage Condition, Specify | Between 0 and 0 *Avoid excessive heat and humidity |
| Special Storage Condition, Specify | Between 0 and 0 *Avoid excessive heat and humidity |
| Special Storage Condition, Specify | Between 0 and 0 *Avoid excessive heat and humidity |
| Special Storage Condition, Specify | Between 0 and 0 *Avoid excessive heat and humidity |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00381780000313 [Unit of Use] |
| GS1 | 10381780000310 [Primary] |
| GS1 | 30381780000314 [Package] Contains: 10381780000310 Package: Case [4 Units] In Commercial Distribution |
| HIBCC | M268ORACOTEPKG101 [Secondary] |
FDA Product Code
| LPG | Material, Dressing, Surgical, Polylactic Acid |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | true |
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 5 |
| Public Version Date | 2020-05-06 |
| Device Publish Date | 2014-09-11 |
Devices Manufactured by Integra Lifesciences Corporation
| 10381780537182 - MAYFIELD® | 2025-09-02 MAYFIELD® Ghost™ Base Unit, Post |
| M24841C14441 - MAYFIELD® | 2025-04-02 Left Horseshoe Gel Pad |
| M24841C14451 - MAYFIELD® | 2025-04-02 Right Horseshoe Gel Pad |
| M248A1008P1 - MAYFIELD® | 2025-04-02 POOL - MAYFIELD GENERAL PURPOSE HEADREST |
| M248A1011P1 - MAYFIELD® | 2025-04-02 POOL- MAYFIELD HORSESHOE HEADREST |
| M248A1011SRL1 - MAYFIELD® | 2025-04-02 MAYFIELD HORSESHOE HEADREST |
| M248A1012P1 - MAYFIELD® | 2025-04-02 POOL - MAYFIELD SWIVEL HORSESHOE HEADREST |
| M248A1012SRL1 - MAYFIELD® | 2025-04-02 MAYFIELD SWIVEL HORSESHOE HEADREST |
Trademark Results [ORACOTE]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
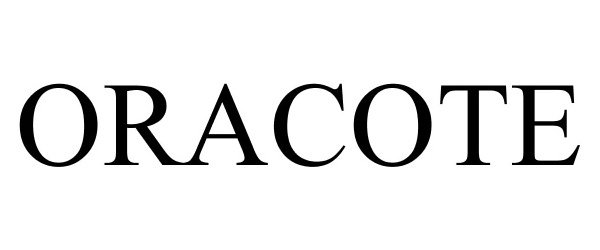 ORACOTE 85306000 4386198 Live/Registered |
Salvin Dental Specialties, Inc. 2011-04-27 |
 ORACOTE 79130124 not registered Dead/Abandoned |
RICERFARMA S.P.A. 2013-03-01 |
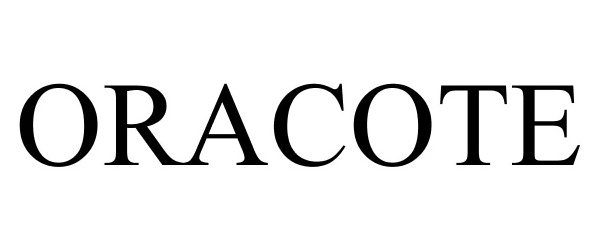 ORACOTE 77788394 not registered Dead/Abandoned |
RICERFARMA S.R.L. 2009-07-23 |
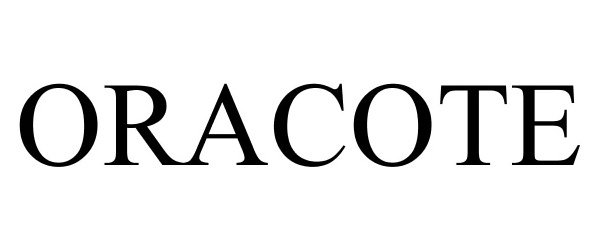 ORACOTE 77778816 not registered Dead/Abandoned |
Zila Pharmaceuticals, Inc. 2009-07-10 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.