Resistex
GUDID 30641043370012
MERCURY ENTERPRISES, INC.
Incentive spirometer| Primary Device ID | 30641043370012 |
| NIH Device Record Key | 11bedcce-9a47-49a1-a2a2-93cd8822edcd |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | Resistex |
| Version Model Number | 10-37001 |
| Company DUNS | 032705659 |
| Company Name | MERCURY ENTERPRISES, INC. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | true |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | true |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 10641043370018 [Primary] |
| GS1 | 30641043370012 [Package] Contains: 10641043370018 Package: [12 Units] In Commercial Distribution |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K942919
FDA Product Code
| BWF | Spirometer, Therapeutic (Incentive) |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 5 |
| Public Version Date | 2020-04-09 |
| Device Publish Date | 2016-03-02 |
On-Brand Devices [Resistex]
| 30641043370012 | 10-37001 |
| 10641043370001 | 10-37000 |
Trademark Results [Resistex]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 RESISTEX 90138799 not registered Live/Pending |
U.S. Glove Co., Inc. 2020-08-26 |
 RESISTEX 85609160 4407926 Live/Registered |
AMCOL International Corporation 2012-04-26 |
 RESISTEX 77106039 not registered Dead/Abandoned |
Sage Products, Inc. 2007-02-13 |
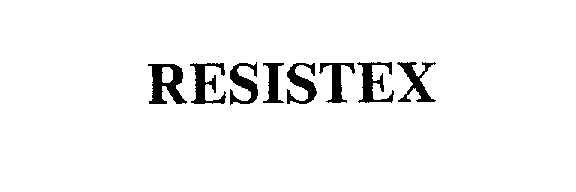 RESISTEX 75692962 2340990 Live/Registered |
PLANTIVA LLC 1999-04-26 |
 RESISTEX 75310346 not registered Dead/Abandoned |
Botanica Laboratories International, Inc. 1997-06-17 |
 RESISTEX 74466868 1864608 Live/Registered |
MERCURY ENTERPRISES, INC. 1993-12-06 |
 RESISTEX 74075345 1659770 Dead/Cancelled |
Parex, Incorporated 1990-07-05 |
 RESISTEX 74071815 1642850 Dead/Cancelled |
American Manufacturing Company, Inc. 1990-06-22 |
 RESISTEX 72097361 0751506 Dead/Expired |
AMERICAN MANUFACTURING COMPANY, INC. 1960-05-18 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.