E-PACK 9849E
GUDID 30705031242467
E-Pack Procedure Kit
ETHICON INC.
General/plastic surgical procedure kit, non-medicated, single-use| Primary Device ID | 30705031242467 |
| NIH Device Record Key | cde12c89-528d-4ed6-9c15-421f5dafd888 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | E-PACK |
| Version Model Number | 9849E |
| Catalog Number | 9849E |
| Company DUNS | 002144145 |
| Company Name | ETHICON INC. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | true |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
| Phone | +1(877)384-4266 |
| xxx@xxx.xxx | |
| Phone | +1(877)384-4266 |
| xxx@xxx.xxx | |
| Phone | +1(877)384-4266 |
| xxx@xxx.xxx | |
| Phone | +1(877)384-4266 |
| xxx@xxx.xxx | |
| Phone | +1(877)384-4266 |
| xxx@xxx.xxx | |
| Phone | +1(877)384-4266 |
| xxx@xxx.xxx | |
| Phone | +1(877)384-4266 |
| xxx@xxx.xxx | |
| Phone | +1(877)384-4266 |
| xxx@xxx.xxx | |
| Phone | +1(877)384-4266 |
| xxx@xxx.xxx | |
| Phone | +1(877)384-4266 |
| xxx@xxx.xxx | |
| Phone | +1(877)384-4266 |
| xxx@xxx.xxx | |
| Phone | +1(877)384-4266 |
| xxx@xxx.xxx | |
| Phone | +1(877)384-4266 |
| xxx@xxx.xxx | |
| Phone | +1(877)384-4266 |
| xxx@xxx.xxx | |
| Phone | +1(877)384-4266 |
| xxx@xxx.xxx | |
| Phone | +1(877)384-4266 |
| xxx@xxx.xxx | |
| Phone | +1(877)384-4266 |
| xxx@xxx.xxx | |
| Phone | +1(877)384-4266 |
| xxx@xxx.xxx | |
| Phone | +1(877)384-4266 |
| xxx@xxx.xxx | |
| Phone | +1(877)384-4266 |
| xxx@xxx.xxx | |
| Phone | +1(877)384-4266 |
| xxx@xxx.xxx |
Operating and Storage Conditions
| Special Storage Condition, Specify | Between 0 and 0 *Away from moisture and direct heat |
| Storage Environment Temperature | Between 0 and 25 Degrees Celsius |
| Storage Environment Temperature | Between 0 and 77 Degrees Fahrenheit |
| Special Storage Condition, Specify | Between 0 and 0 *Away from moisture and direct heat |
| Storage Environment Temperature | Between 0 and 25 Degrees Celsius |
| Storage Environment Temperature | Between 0 and 77 Degrees Fahrenheit |
| Special Storage Condition, Specify | Between 0 and 0 *Away from moisture and direct heat |
| Storage Environment Temperature | Between 0 and 77 Degrees Fahrenheit |
| Storage Environment Temperature | Between 0 and 25 Degrees Celsius |
| Special Storage Condition, Specify | Between 0 and 0 *Away from moisture and direct heat |
| Storage Environment Temperature | Between 0 and 77 Degrees Fahrenheit |
| Storage Environment Temperature | Between 0 and 25 Degrees Celsius |
| Special Storage Condition, Specify | Between 0 and 0 *Away from moisture and direct heat |
| Storage Environment Temperature | Between 0 and 77 Degrees Fahrenheit |
| Storage Environment Temperature | Between 0 and 25 Degrees Celsius |
| Special Storage Condition, Specify | Between 0 and 0 *Away from moisture and direct heat |
| Storage Environment Temperature | Between 0 and 77 Degrees Fahrenheit |
| Storage Environment Temperature | Between 0 and 25 Degrees Celsius |
| Special Storage Condition, Specify | Between 0 and 0 *Away from moisture and direct heat |
| Storage Environment Temperature | Between 0 and 77 Degrees Fahrenheit |
| Storage Environment Temperature | Between 0 and 25 Degrees Celsius |
| Special Storage Condition, Specify | Between 0 and 0 *Away from moisture and direct heat |
| Storage Environment Temperature | Between 0 and 77 Degrees Fahrenheit |
| Storage Environment Temperature | Between 0 and 25 Degrees Celsius |
| Special Storage Condition, Specify | Between 0 and 0 *Away from moisture and direct heat |
| Storage Environment Temperature | Between 0 and 77 Degrees Fahrenheit |
| Storage Environment Temperature | Between 0 and 25 Degrees Celsius |
| Special Storage Condition, Specify | Between 0 and 0 *Away from moisture and direct heat |
| Storage Environment Temperature | Between 0 and 77 Degrees Fahrenheit |
| Storage Environment Temperature | Between 0 and 25 Degrees Celsius |
| Special Storage Condition, Specify | Between 0 and 0 *Away from moisture and direct heat |
| Storage Environment Temperature | Between 0 and 77 Degrees Fahrenheit |
| Storage Environment Temperature | Between 0 and 25 Degrees Celsius |
| Special Storage Condition, Specify | Between 0 and 0 *Away from moisture and direct heat |
| Storage Environment Temperature | Between 0 and 77 Degrees Fahrenheit |
| Storage Environment Temperature | Between 0 and 25 Degrees Celsius |
| Special Storage Condition, Specify | Between 0 and 0 *Away from moisture and direct heat |
| Storage Environment Temperature | Between 0 and 77 Degrees Fahrenheit |
| Storage Environment Temperature | Between 0 and 25 Degrees Celsius |
| Special Storage Condition, Specify | Between 0 and 0 *Away from moisture and direct heat |
| Storage Environment Temperature | Between 0 and 77 Degrees Fahrenheit |
| Storage Environment Temperature | Between 0 and 25 Degrees Celsius |
| Special Storage Condition, Specify | Between 0 and 0 *Away from moisture and direct heat |
| Storage Environment Temperature | Between 0 and 77 Degrees Fahrenheit |
| Storage Environment Temperature | Between 0 and 25 Degrees Celsius |
| Special Storage Condition, Specify | Between 0 and 0 *Away from moisture and direct heat |
| Storage Environment Temperature | Between 0 and 77 Degrees Fahrenheit |
| Storage Environment Temperature | Between 0 and 25 Degrees Celsius |
| Special Storage Condition, Specify | Between 0 and 0 *Away from moisture and direct heat |
| Storage Environment Temperature | Between 0 and 77 Degrees Fahrenheit |
| Storage Environment Temperature | Between 0 and 25 Degrees Celsius |
| Special Storage Condition, Specify | Between 0 and 0 *Away from moisture and direct heat |
| Storage Environment Temperature | Between 0 and 77 Degrees Fahrenheit |
| Storage Environment Temperature | Between 0 and 25 Degrees Celsius |
| Special Storage Condition, Specify | Between 0 and 0 *Away from moisture and direct heat |
| Storage Environment Temperature | Between 0 and 77 Degrees Fahrenheit |
| Storage Environment Temperature | Between 0 and 25 Degrees Celsius |
| Special Storage Condition, Specify | Between 0 and 0 *Away from moisture and direct heat |
| Storage Environment Temperature | Between 0 and 77 Degrees Fahrenheit |
| Storage Environment Temperature | Between 0 and 25 Degrees Celsius |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 20705031242460 [Primary] |
| GS1 | 30705031242467 [Package] Contains: 20705031242460 Package: BOX [5 Units] In Commercial Distribution |
FDA Product Code
| GAM | SUTURE, ABSORBABLE, SYNTHETIC, POLYGLYCOLIC ACID |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | true |
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 3 |
| Public Version Date | 2018-05-08 |
| Device Publish Date | 2017-12-06 |
On-Brand Devices [E-PACK]
| 30705031245833 | E-Pack Procedure Kit |
| 30705031245826 | E-Pack Procedure Kit |
| 30705031245741 | E-Pack Procedure Kit |
| 30705031245734 | E-Pack Procedure Kit |
| 30705031245710 | E-Pack Procedure Kit |
| 30705031245703 | E-Pack Procedure Kit |
| 30705031245697 | E-Pack Procedure Kit |
| 30705031245505 | E-Pack Procedure Kit |
| 30705031245499 | E-Pack Procedure Kit |
| 30705031245482 | E-Pack Procedure Kit |
| 30705031245475 | E-Pack Procedure Kit |
| 30705031245468 | E-Pack Procedure Kit |
| 30705031245451 | E-Pack Procedure Kit |
| 30705031245444 | E-Pack Procedure Kit |
| 30705031243730 | E-Pack Procedure Kit |
| 30705031243655 | E-Pack Procedure Kit |
| 30705031243389 | E-Pack Procedure Kit |
| 30705031243259 | E-Pack Procedure Kit |
| 30705031243235 | E-Pack Procedure Kit |
| 30705031243167 | E-Pack Procedure Kit |
| 30705031242863 | E-Pack Procedure Kit |
| 30705031242856 | E-Pack Procedure Kit |
| 30705031242849 | E-Pack Procedure Kit |
| 30705031242832 | E-Pack Procedure Kit |
| 30705031242825 | E-Pack Procedure Kit |
| 30705031242818 | E-Pack Procedure Kit |
| 30705031242801 | E-Pack Procedure Kit |
| 30705031242528 | E-Pack Procedure Kit |
| 30705031242504 | E-Pack Procedure Kit |
| 30705031242498 | E-Pack Procedure Kit |
| 30705031242481 | E-Pack Procedure Kit |
| 30705031242467 | E-Pack Procedure Kit |
| 30705031242450 | E-Pack Procedure Kit |
| 30705031242443 | E-Pack Procedure Kit |
| 30705031242436 | E-Pack Procedure Kit |
| 30705031242429 | E-Pack Procedure Kit |
| 30705031242412 | E-Pack Procedure Kit |
| 30705031242405 | E-Pack Procedure Kit |
| 30705031242399 | E-Pack Procedure Kit |
| 30705031242382 | E-Pack Procedure Kit |
| 30705031242375 | E-Pack Procedure Kit |
| 30705031242368 | E-Pack Procedure Kit |
| 30705031242351 | E-Pack Procedure Kit |
| 30705031242344 | E-Pack Procedure Kit |
| 30705031242337 | E-Pack Procedure Kit |
| 30705031242320 | E-Pack Procedure Kit |
| 30705031242313 | E-Pack Procedure Kit |
| 30705031242306 | E-Pack Procedure Kit |
| 30705031242023 | E-Pack Procedure Kit |
| 30705031242016 | E-Pack Procedure Kit |
Trademark Results [E-PACK]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 E-PACK 97072428 not registered Live/Pending |
Tadano Ltd. 2021-10-13 |
 E-PACK 87775282 not registered Live/Pending |
The Upper Deck Company 2018-01-29 |
 E-PACK 78372383 not registered Dead/Abandoned |
Vicuron Pharmaceuticals Inc. 2004-02-23 |
 E-PACK 78050843 not registered Dead/Abandoned |
Winmark Concepts, Inc. 2001-03-01 |
 E-PACK 76529359 2862350 Live/Registered |
THOMAS EDISON STATE UNIVERSITY 2003-07-14 |
 E-PACK 76125736 2847129 Dead/Cancelled |
BHS GETRIEBE GMBH 2000-09-11 |
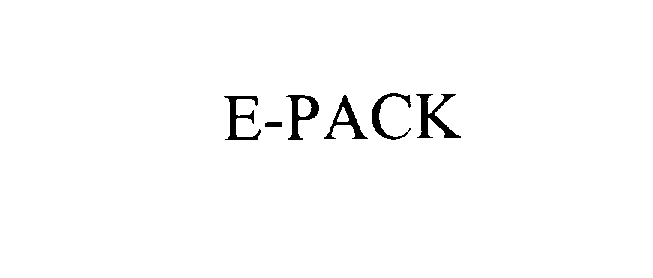 E-PACK 76125736 2847129 Dead/Cancelled |
BHS GETRIEBE GMBH 2000-09-11 |
 E-PACK 76080504 2621994 Live/Registered |
CNA FINANCIAL CORPORATION 2000-06-29 |
 E-PACK 75617808 not registered Dead/Abandoned |
e-pack LLC 1999-01-11 |
 E-PACK 75530999 not registered Dead/Abandoned |
UMI Company 1998-08-05 |
 E-PACK 73573363 1412175 Live/Registered |
JOHNSON & JOHNSON 1985-12-13 |
 E-PACK 72306115 0882854 Dead/Cancelled |
PATENT DEVELOPMENT ASSOCIATES, INC. 1968-08-28 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.