Pro-Line® CT
GUDID 30884908094276
6F X 60CM DUAL LUMEN Pro-Line® CT W/ CUFF @ 2CM BASIC IR SET
Medical Components, Inc.
Peripherally-inserted central venous catheter| Primary Device ID | 30884908094276 |
| NIH Device Record Key | bee1fd4a-bade-4639-b9d4-235eae01875c |
| Commercial Distribution Discontinuation | 2019-12-02 |
| Commercial Distribution Status | Not in Commercial Distribution |
| Brand Name | Pro-Line® CT |
| Version Model Number | MR28036221 |
| Company DUNS | 038000253 |
| Company Name | Medical Components, Inc. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | true |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | true |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
| Phone | +1(215)256-4201 |
| clinical@medcompnet.com | |
| Phone | +1(215)256-4201 |
| clinical@medcompnet.com | |
| Phone | +1(215)256-4201 |
| clinical@medcompnet.com | |
| Phone | +1(215)256-4201 |
| clinical@medcompnet.com | |
| Phone | +1(215)256-4201 |
| clinical@medcompnet.com | |
| Phone | +1(215)256-4201 |
| clinical@medcompnet.com | |
| Phone | +1(215)256-4201 |
| clinical@medcompnet.com | |
| Phone | +1(215)256-4201 |
| clinical@medcompnet.com | |
| Phone | +1(215)256-4201 |
| clinical@medcompnet.com | |
| Phone | +1(215)256-4201 |
| clinical@medcompnet.com | |
| Phone | +1(215)256-4201 |
| clinical@medcompnet.com | |
| Phone | +1(215)256-4201 |
| clinical@medcompnet.com | |
| Phone | +1(215)256-4201 |
| clinical@medcompnet.com | |
| Phone | +1(215)256-4201 |
| clinical@medcompnet.com | |
| Phone | +1(215)256-4201 |
| clinical@medcompnet.com | |
| Phone | +1(215)256-4201 |
| clinical@medcompnet.com | |
| Phone | +1(215)256-4201 |
| clinical@medcompnet.com |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00884908094275 [Primary] |
| GS1 | 30884908094276 [Package] Contains: 50884908094270 Package: inner box [1 Units] Discontinued: 2019-12-02 Not in Commercial Distribution |
| GS1 | 50884908094270 [Package] Contains: 00884908094275 Package: box [5 Units] Discontinued: 2019-12-02 Not in Commercial Distribution |
FDA Product Code
| LJS | Catheter,intravascular,therapeutic,long-term greater than 30 days |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | true |
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 3 |
| Public Version Date | 2019-12-04 |
| Device Publish Date | 2015-10-22 |
On-Brand Devices [Pro-Line® CT]
| 50884908107475 | 5F X 55CM DUAL LUMEN Pro-Line® CT W/CUFF @ 2CM BASIC IR SET |
| 50884908107468 | 5F X 55CM DUAL LUMEN Pro-Line® CT W/CUFF @ 5CM BASIC IR SET |
| 50884908105051 | 6F X 60CM TRIPLE Pro-Line® CT OR SET W/0.038" WIRE |
| 50884908104931 | 7F X 60CM DUAL PRO-LINE® CT OR SET W/ .038 WIRE |
| 50884908104870 | 7F X 60CM SINGLE PRO-LINE® CT OR SET W/ .038" WIRE |
| 50884908104863 | 6F X 60CM DUAL Pro-Line® CT OR SET W/ .038" WIRE |
| 30884908104852 | 6F X 60CM SINGLE PRO-LINE® CT OR SET W/ .038" WIRE |
| 50884908097752 | 6F X 60CM TRIPLE LUMEN Pro-Line® CT BASIC IR SET |
| 50884908097172 | 7F X 60CM DUAL Pro-Line® CT BASIC IR SET |
| 50884908097165 | 7F X 60CM SINGLE Pro-Line® CT BASIC IR SET |
| 30884908094283 | 6F X 60CM SINGLE LUMEN Pro-Line® CT W/ CUFF @ 2CM BASIC IR SET |
| 30884908094276 | 6F X 60CM DUAL LUMEN Pro-Line® CT W/ CUFF @ 2CM BASIC IR SET |
| 50884908094263 | 5F X 60CM SINGLE LUMEN Pro-Line® CT w/ CUFF @2CM BASIC IR SET |
| 50884908039226 | 6F X 60CM DUAL PRO-LINE® CT BASIC IR SET |
| 50884908034641 | 7F X 60CM DUAL LUMEN PRO-LINE® CT BASIC IR SET |
| 50884908034634 | 7F X 60CM SINGLE LUMEN Pro-Line® CT BASIC IR SET |
| 30884908034623 | 6F X 60CM DUAL LUMEN Pro-Line® CT W/CUFF @ 5CM BASIC IR SET |
| 50884908034610 | 6F X 60CM SINGLE LUMEN Pro-Line® CT BASIC IR SET |
| 50884908034603 | 5F X 60CM SINGLE LUMEN Pro-Line® CT BASIC IR SET |
| 50884908180614 | 7F X 60CM DUAL Pro-Line® CT BASIC SET |
| 50884908180607 | 7F X 60CM SINGLE Pro-Line® CT BASIC SET |
| 50884908143930 | 6F X 60CM SINGLE PRO-LINE® CT OR SET W/0.038" WIRE |
| 50884908143923 | 7F X 60CM DUAL Pro-Line® CT BASIC SET |
| 50884908130725 | 7F X 60CM SINGLE Pro-Line® CT BASIC SET |
| 50884908062064 | 6F X 60CM DUAL Pro-Line® CT OR SET W/0.038" WIRE |
| 50884908062132 | 7F X 60CM DUAL PRO-LINE® CT OR SET W/0.038" WIRE |
| 50884908062101 | 7F X 60CM SINGLE PRO-LINE® CT OR SET W/0.038" WIRE |
Trademark Results [Pro-Line]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
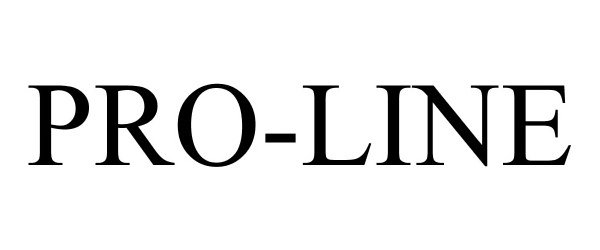 PRO-LINE 87886047 5637047 Live/Registered |
D.T. Mattson Enterprises, Inc. 2018-04-20 |
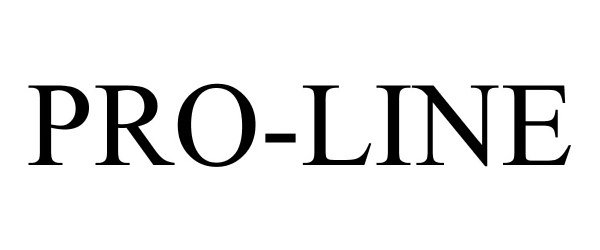 PRO-LINE 86482307 5020095 Live/Registered |
CQT KENNEDY, LLC 2014-12-16 |
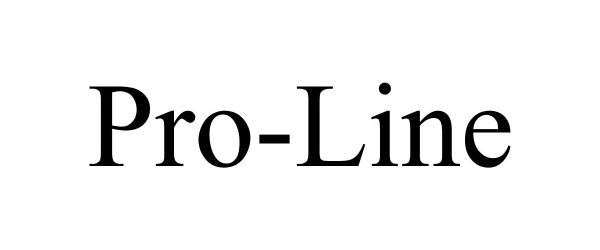 PRO-LINE 85802453 4419898 Live/Registered |
elite grips USA Inc. 2012-12-13 |
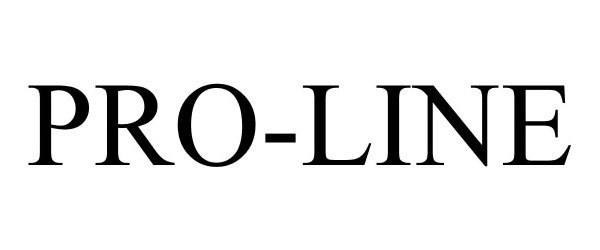 PRO-LINE 85776109 4361290 Live/Registered |
Pro Line Distributing, Inc. 2012-11-09 |
 PRO-LINE 85122256 3957361 Dead/Cancelled |
D.T. MATTSON ENTERPRISES, INC. 2010-09-02 |
 PRO-LINE 78171523 not registered Dead/Abandoned |
Pro-Line International, Inc. 2002-10-07 |
 PRO-LINE 77284233 3504002 Live/Registered |
Pro-Line Performance, Inc. 2007-09-20 |
 PRO-LINE 76667309 3521029 Dead/Cancelled |
Medical Components, Inc. 2006-10-12 |
 PRO-LINE 76522806 not registered Dead/Abandoned |
DT MATTSON ENTERPRISES, INC. 2003-06-16 |
 PRO-LINE 76373872 2744495 Live/Registered |
LA-CO INDUSTRIES, INC. 2002-02-21 |
 PRO-LINE 76336366 2731351 Dead/Cancelled |
Mulholland Headwear, Ltd. 2001-11-13 |
 PRO-LINE 76277373 3097018 Dead/Cancelled |
L & P Property Management Company 2001-06-27 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.