SuperNO2VA SNL-20
GUDID 30889483605985
SuperNO2VA Nasal PAP Ventilation Device, Large
Sunmed Group Holdings, LLC
CPAP/BPAP nasal mask, single-use| Primary Device ID | 30889483605985 |
| NIH Device Record Key | ba01ca7e-07be-4a3c-b93a-acd56202ea6b |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | SuperNO2VA |
| Version Model Number | SNL-20 |
| Catalog Number | SNL-20 |
| Company DUNS | 119058668 |
| Company Name | Sunmed Group Holdings, LLC |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | true |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
| Phone | (800)433-2797 |
| info@sun-med.com | |
| Phone | (800)433-2797 |
| info@sun-med.com | |
| Phone | (800)433-2797 |
| info@sun-med.com | |
| Phone | (800)433-2797 |
| info@sun-med.com | |
| Phone | (800)433-2797 |
| info@sun-med.com | |
| Phone | (800)433-2797 |
| info@sun-med.com | |
| Phone | (800)433-2797 |
| info@sun-med.com | |
| Phone | (800)433-2797 |
| info@sun-med.com | |
| Phone | (800)433-2797 |
| info@sun-med.com | |
| Phone | (800)433-2797 |
| info@sun-med.com | |
| Phone | (800)433-2797 |
| info@sun-med.com | |
| Phone | (800)433-2797 |
| info@sun-med.com | |
| Phone | (800)433-2797 |
| info@sun-med.com | |
| Phone | (800)433-2797 |
| info@sun-med.com | |
| Phone | (800)433-2797 |
| info@sun-med.com | |
| Phone | (800)433-2797 |
| info@sun-med.com | |
| Phone | (800)433-2797 |
| info@sun-med.com | |
| Phone | (800)433-2797 |
| info@sun-med.com | |
| Phone | (800)433-2797 |
| info@sun-med.com | |
| Phone | (800)433-2797 |
| info@sun-med.com | |
| Phone | (800)433-2797 |
| info@sun-med.com | |
| Phone | (800)433-2797 |
| info@sun-med.com | |
| Phone | (800)433-2797 |
| info@sun-med.com | |
| Phone | (800)433-2797 |
| info@sun-med.com | |
| Phone | (800)433-2797 |
| info@sun-med.com | |
| Phone | (800)433-2797 |
| info@sun-med.com | |
| Phone | (800)433-2797 |
| info@sun-med.com |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 10190752145450 [Previous] |
| GS1 | 10889483605981 [Primary] |
| GS1 | 30889483605985 [Package] Contains: 10889483605981 Package: Case [20 Units] In Commercial Distribution |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K163277
FDA Product Code
| BSJ | Mask, Gas, Anesthetic |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2025-02-27 |
| Device Publish Date | 2025-02-19 |
On-Brand Devices [SuperNO2VA]
| 30889483606043 | SuperNO2VA ET SYSTEM, Adult Medium |
| 30889483606029 | SuperNO2VA ET SYSTEM, Adult Large |
| 30889483166301 | SuperNO2VA Nasal PAP Ventilation System, Medium |
| 30889483166295 | SuperNO2VA Nasal PAP Ventilation System, Large |
| 30889483605992 | SuperNO2VA Nasal PAP Ventilation Device, Medium |
| 30889483605985 | SuperNO2VA Nasal PAP Ventilation Device, Large |
| 30889483605688 | SuperNO2VA Et Mask, Adult Medium |
| 30889483605664 | SuperNO2VA Et Mask, Adult Large |
| 30889483606050 | SuperNO2VA Et Mask, Adult Medium |
| 30889483606036 | SuperNO2VA Et Mask, Adult Large |
| 30889483605695 | SuperNO2VA Et Mask, Adult Medium |
| 30889483605671 | SuperNO2VA Et Mask, Adult Large |
Trademark Results [SuperNO2VA]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
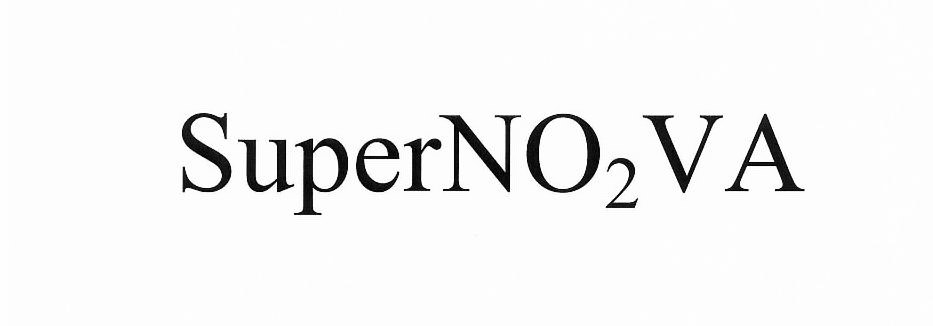 SUPERNO2VA 86414780 4919015 Live/Registered |
REVOLUTIONARY MEDICAL DEVICES, INC. 2014-10-03 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.