IRIS 0220180518
GUDID 37613327174817
[Ureteral Kit: 2 - Ureteral Catheters, 6 Fr Outer Diameter, 70 cm Length, 2 - Emitting Fiber, 0.75 Outer Diameter, 370 cm Length, Do not use if pack
STRYKER CORPORATION
Ureteral transilluminator, single-use| Primary Device ID | 37613327174817 |
| NIH Device Record Key | 6444a0fd-a4dd-43dc-bb3c-25679bb7c6d2 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | IRIS |
| Version Model Number | 0220180518 |
| Catalog Number | 0220180518 |
| Company DUNS | 187502109 |
| Company Name | STRYKER CORPORATION |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
| Phone | +1(866)624-4422 |
| xx@xx.xx |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 07613327174816 [Primary] |
| GS1 | 37613327174817 [Package] Contains: 07613327174816 Package: pack [5 Units] In Commercial Distribution |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K151243
FDA Product Code
| FCW | Light source, fiberoptic, routine |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | true |
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 4 |
| Public Version Date | 2019-02-07 |
| Device Publish Date | 2016-09-23 |
Devices Manufactured by STRYKER CORPORATION
| 07613327261844 - O.I.C. TITANIUM | 2026-02-09 CAGE IMPACTOR |
| 07613327261868 - O.I.C. TITANIUM | 2026-02-09 FINAL CAGE IMPACTOR |
| 07613327264722 - XIA 4.5 | 2026-02-09 HOOK IMPACTOR |
| 07613327264753 - XIA 4.5 | 2026-02-09 DUAL STAPLE IMPACTOR |
| 07613327265460 - XIA 3 | 2026-02-09 HOOK IMPACTOR |
| 07613327267037 - VLIFT | 2026-02-09 ENDCAP IMPACTOR |
| 07613327267068 - VLIFT | 2026-02-09 ENDCAP IMPACTOR |
| 07613327267563 - AVS | 2026-02-09 AS SPACER IMPACTOR STRAIGHT |
Trademark Results [IRIS]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 IRIS 98771505 not registered Live/Pending |
Iris Medical Technologies, LLC 2024-09-26 |
 IRIS 98771478 not registered Live/Pending |
Iris Medical Technologies, LLC 2024-09-26 |
 IRIS 98751495 not registered Live/Pending |
SCAN4ALL 2024-09-15 |
 IRIS 98719013 not registered Live/Pending |
THE KENDALL GROUP, INC. 2024-08-27 |
 IRIS 98695508 not registered Live/Pending |
Cascade Maverik Lacrosse, LLC 2024-08-13 |
 IRIS 98674850 not registered Live/Pending |
Respiree Pte Ltd 2024-07-30 |
 IRIS 98643060 not registered Live/Pending |
HERNANDEZ ALONSO, GABRIEL 2024-07-11 |
 IRIS 98634961 not registered Live/Pending |
AVIANA GLOBAL TECHNOLOGIES, INC. 2024-07-05 |
 IRIS 98622280 not registered Live/Pending |
Tennant Company 2024-06-27 |
 IRIS 98622015 not registered Live/Pending |
Tennant Company 2024-06-27 |
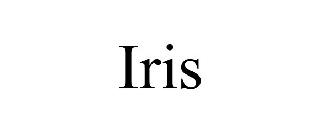 IRIS 98595581 not registered Live/Pending |
CROMetrics 2024-06-11 |
 IRIS 98589845 not registered Live/Pending |
National Service Alliance, LLC 2024-06-07 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.