Remote Control 017380
GUDID 37630039300372
EmpowerMR 17" Remote 1722
Bracco Injeneering SA
Contrast medium injection system control unit| Primary Device ID | 37630039300372 |
| NIH Device Record Key | 157739b9-f376-4ad5-b2b4-ce865d6097d8 |
| Commercial Distribution Status | In Commercial Distribution |
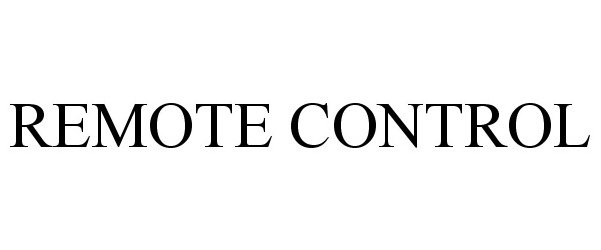
| Brand Name | Remote Control |
| Version Model Number | 017380 |
| Catalog Number | 017380 |
| Company DUNS | 482489007 |
| Company Name | Bracco Injeneering SA |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | false |
| Serial Number | true |
| Manufacturing Date | true |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
| Phone | +41216236030 |
| maud.giorgi@bracco.com | |
| Phone | +41216236030 |
| maud.giorgi@bracco.com |
Operating and Storage Conditions
| Special Storage Condition, Specify | Between 0 and 0 *Dry environment |
| Special Storage Condition, Specify | Between 0 and 0 *Dry environment |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 17630039300378 [Primary] |
| GS1 | 37630039300372 [Package] Contains: 17630039300378 Package: Box [1 Units] In Commercial Distribution |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K062449
FDA Product Code
| IZQ | Injector, Contrast Medium, Automatic |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 3 |
| Public Version Date | 2018-07-06 |
| Device Publish Date | 2016-09-20 |
On-Brand Devices [Remote Control]
| 37630039300785 | EmpowerMR 17" Remote 1721 |
| 37630039300471 | EmpowerCTA+ 17" Remote 1722 |
| 37630039300372 | EmpowerMR 17" Remote 1722 |
| 37630039301034 | Empower MR Remote Control - 1731 |
| 37630039301416 | EmpowerCTA+ 17" Remote 1721 |
| 37630039300983 | EmpowerCTA+ 17" Remote 1731 |
| 37630039302048 | EmpowerCTA+ Injector System, Remote Control |
Trademark Results [Remote Control]
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.