LIFE WORKS LW0006
GUDID 46976040710273
Health Plus (Shanghai) International Co., LTD
Hot/cold therapy pack, single-use| Primary Device ID | 46976040710273 |
| NIH Device Record Key | f226da86-5ed0-4b13-8ae4-381c4bdf48c9 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | LIFE WORKS |
| Version Model Number | LW0006 |
| Catalog Number | LW0006 |
| Company DUNS | 554477472 |
| Company Name | Health Plus (Shanghai) International Co., LTD |
| Device Count | 25 |
| DM Exempt | false |
| Pre-market Exempt | true |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | true |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | false |
| OTC Over-The-Counter | true |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 06976040710275 [Unit of Use] |
| GS1 | 26976040710279 [Primary] |
| GS1 | 46976040710273 [Package] Contains: 26976040710279 Package: CASE [4 Units] In Commercial Distribution |
FDA Product Code
| IMD | Pack, Hot Or Cold, Disposable |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2024-12-16 |
| Device Publish Date | 2024-12-08 |
On-Brand Devices [LIFE WORKS]
| 46976040710273 | LW0006 |
| 36976040710276 | LW0005 |
Trademark Results [LIFE WORKS]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 LIFE WORKS 98718627 not registered Live/Pending |
Health Plus (Shanghai) International Co., Ltd 2024-08-27 |
 LIFE WORKS 97229812 not registered Live/Pending |
GloveCo Holdings, LLC. 2022-01-20 |
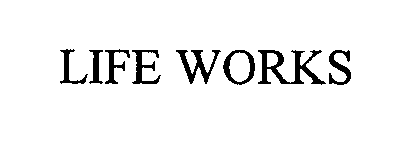 LIFE WORKS 76408775 2681035 Dead/Cancelled |
Life Works, Inc. 2002-05-16 |
 LIFE WORKS 75776890 not registered Dead/Abandoned |
MARCUS, SUSAN J. 1999-08-18 |
 LIFE WORKS 75539291 not registered Dead/Abandoned |
Gold Investment, Inc. 1998-08-19 |
 LIFE WORKS 74447608 1895537 Dead/Cancelled |
OnTrend Enterprises, Inc. 1993-10-15 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.