Sklar
GUDID 50649111440173
PEDERSON SPEC XS 2.75X.5 INCHESCS/25
SKLAR CORPORATION
Vaginal speculum, single-use| Primary Device ID | 50649111440173 |
| NIH Device Record Key | be36e5ca-231f-481e-a8d9-b29f70d6e90a |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | Sklar |
| Version Model Number | 96-2373M |
| Company DUNS | 006966006 |
| Company Name | SKLAR CORPORATION |
| Device Count | 25 |
| DM Exempt | false |
| Pre-market Exempt | true |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | true |
| Manufacturing Date | false |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | true |
| RX Perscription | false |
| OTC Over-The-Counter | false |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00649111440178 [Unit of Use] |
| GS1 | 50649111440173 [Primary] |
FDA Product Code
| HDF | SPECULUM, VAGINAL, METAL |
Sterilization
| Steralize Prior To Use | true |
| Device Is Sterile | false |
[50649111440173]
Ethylene Oxide
[50649111440173]
Ethylene Oxide
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2019-03-28 |
| Device Publish Date | 2019-03-20 |
On-Brand Devices [Sklar]
| 50649111440869 | GRAVES SPEC XLG4.75X1.5 CS/25 |
| 50649111440852 | GRAVES SPEC XLG4.75X1.5 CS/25 |
| 50649111440845 | GRAVES SPEC LG 1 3/8X4.5 INCHESCS/25 |
| 50649111440838 | GRAVES VAG SPEC MD1.25X4 INCHESCS/25 |
| 50649111440821 | GRAVES VAG SPEC SM.75 X3 INCHESCS/25 |
| 50649111440685 | STRABISMUS SCISS STR4.5 INCHESCS/25 |
| 50649111440647 | IRIS SCISS STR S/S 3.5 INCHES CS/25 |
| 50649111440630 | IRIS SCISS STR S/S 3.5 INCHES CS/25 |
| 50649111440623 | OPER SCISS 6.5CV S/B STRLCS/25 |
| 50649111440616 | OPER SCISS 6.5CV S/B STRLCS/25 |
| 50649111440531 | IRIS SCISS CVD S/S 3.5 INCHES CS/25 |
| 50649111440524 | IRIS SCISS CVD S/S 3.5 INCHES CS/25 |
| 50649111440401 | METZ-NELSON SCISS CVD 9 INCHESCS/25 |
| 50649111440395 | METZ-NELSON SCISS CVD 9 INCHES CS/25 |
| 50649111440388 | MAYO DISS SCISS CVD 6.75 INCHESCS/25 |
| 50649111440371 | MAYO DISS SCISS CVD 6.75 INCHESCS/25 |
| 50649111440364 | MAYO DISS SCISS CVD 5.5 INCHESCS/25 |
| 50649111440357 | MAYO DISS SCISS CVD 5.5 INCHESCS/25 |
| 50649111440302 | OPER SCISS 4.5 INCHESCV SS STRLCS/25 |
| 50649111440296 | OPER SCISS 4.5 INCHES CVD SS CS/25 |
| 50649111440227 | PEDERSN SPEC XLG4.75X15/8CS/25 |
| 50649111440210 | PEDERSN SPEC XLG4.75X15/8CS/25 |
| 50649111440203 | PEDERSON VAGSPEC LG1X4.75CS/25 |
| 50649111440197 | PEDERSON VAG SPEC MD7/8X4CS/25 |
| 50649111440180 | PEDERSON VAG SPEC SM.5X3 INCHESCS/25 |
| 50649111440173 | PEDERSON SPEC XS 2.75X.5 INCHESCS/25 |
| 50649111440166 | PEDERSON SPEC XS 2.75X.5 INCHESCS/25 |
| 50649111440159 | PEDERSON SPEC SLENDR STRLCS/25 |
| 50649111439900 | METZENBAUM SCIS STR7 INCHESSTRLCS/25 |
| 50649111439894 | METZENBAUM SCIS STR7 INCHESSTRLCS/25 |
| 50649111439887 | MAYO DISS SCISS CV 9 INCHESSTRLCS/25 |
| 50649111439870 | MAYO DISS SCISS CV 9 INCHESSTRLCS/25 |
| 50649111439863 | OP SCISS STR B/B 5.5 INCHESSTRLCS/25 |
| 50649111439856 | OP SCISS STR B/B 5.5 INCHESSTRLCS/25 |
| 50649111439849 | OPER SCISSOR CV B/B 4.5 INCHES CS/25 |
| 50649111439832 | OPER SCISSOR CV B/B 4.5 INCHES CS/25 |
| 50649111439788 | VIENNA NSL SPEC LG STRL CS/25 |
| 50649111439771 | VIENNA NSL SPEC LG STRL CS/25 |
| 50649111439764 | VIENNA NSL SPEC MD STRL CS/25 |
| 50649111439757 | VIENNA NSL SPEC MD STRL CS/25 |
| 50649111400610 | FN-PT SPLINT FCP 4 1/2 STER 50 |
| 50649111400603 | TUBE OCC FCP SM STR 7 INCHES STER 25 |
| 50649111400597 | TUBE OCCL FCP SM STR 7 INCHESSTER 50 |
| 50649111357846 | SKLAR DISINFECTANT WIPES CS/12 |
| 50649111355989 | SKLAR SOAK DECONT 1 GAL CS/4 |
| 50649111268326 | NH SMOOTH 5.25 WIREFORM CS/50 |
| 50649111152236 | PLAS TOWEL CLAMP STER CS/50 |
| 50649111035195 | TUBE OCCL FCP SM STR 7 INCHESSTER 50 |
| 40649111401023 | PLAST SPONGE FCP 7 1/4 INCHES BX/100 |
| 40649111162672 | NASAL SPEC DISP 5 1/2 INCHES BX/48 |
Trademark Results [Sklar]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
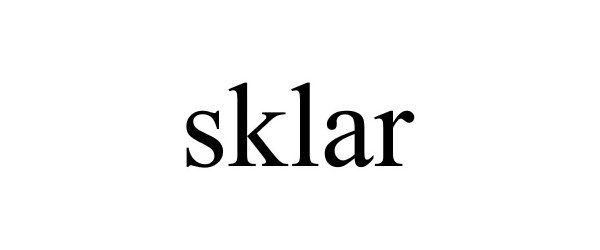 SKLAR 85264381 4148729 Live/Registered |
Sklar Corporation 2011-03-11 |
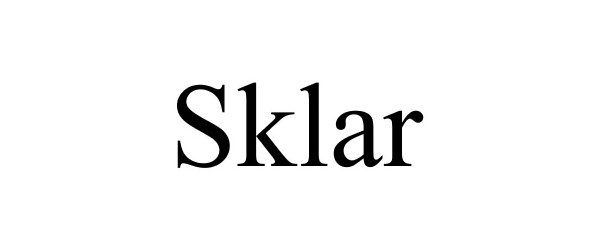 SKLAR 77889478 not registered Dead/Abandoned |
Sklar Corporation 2009-12-09 |
 SKLAR 72017598 0649405 Dead/Expired |
J. SKLAR MANUFACTURING CO., INC. 1956-10-16 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.