PRO-LINE® CT PRESSURE INJECTABLE CVC (ISOPLAST) MD28036201
GUDID 50884908155988
6F X 60CM DUAL LUMEN PRO-LINE® CT W/CUFF @ 5CM BASIC IR SET
Medical Components, Inc.
Centrally-inserted central venous catheter| Primary Device ID | 50884908155988 |
| NIH Device Record Key | a776a338-d3fe-4231-8dff-dec0ccdb1d5a |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | PRO-LINE® CT PRESSURE INJECTABLE CVC (ISOPLAST) |
| Version Model Number | MD28036201 |
| Catalog Number | MD28036201 |
| Company DUNS | 038000253 |
| Company Name | Medical Components, Inc. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | true |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | true |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
| Phone | +1(215)256-4201 |
| clinical@medcompnet.com | |
| Phone | +1(215)256-4201 |
| clinical@medcompnet.com | |
| Phone | +1(215)256-4201 |
| clinical@medcompnet.com | |
| Phone | +1(215)256-4201 |
| clinical@medcompnet.com | |
| Phone | +1(215)256-4201 |
| clinical@medcompnet.com | |
| Phone | +1(215)256-4201 |
| clinical@medcompnet.com | |
| Phone | +1(215)256-4201 |
| clinical@medcompnet.com | |
| Phone | +1(215)256-4201 |
| clinical@medcompnet.com | |
| Phone | +1(215)256-4201 |
| clinical@medcompnet.com | |
| Phone | +1(215)256-4201 |
| clinical@medcompnet.com | |
| Phone | +1(215)256-4201 |
| clinical@medcompnet.com | |
| Phone | +1(215)256-4201 |
| clinical@medcompnet.com | |
| Phone | +1(215)256-4201 |
| clinical@medcompnet.com | |
| Phone | +1(215)256-4201 |
| clinical@medcompnet.com | |
| Phone | +1(215)256-4201 |
| clinical@medcompnet.com | |
| Phone | +1(215)256-4201 |
| clinical@medcompnet.com | |
| Phone | +1(215)256-4201 |
| clinical@medcompnet.com | |
| Phone | +1(215)256-4201 |
| clinical@medcompnet.com | |
| Phone | +1(215)256-4201 |
| clinical@medcompnet.com | |
| Phone | +1(215)256-4201 |
| clinical@medcompnet.com | |
| Phone | +1(215)256-4201 |
| clinical@medcompnet.com | |
| Phone | +1(215)256-4201 |
| clinical@medcompnet.com | |
| Phone | +1(215)256-4201 |
| clinical@medcompnet.com | |
| Phone | +1(215)256-4201 |
| clinical@medcompnet.com | |
| Phone | +1(215)256-4201 |
| clinical@medcompnet.com | |
| Phone | +1(215)256-4201 |
| clinical@medcompnet.com | |
| Phone | +1(215)256-4201 |
| clinical@medcompnet.com | |
| Phone | +1(215)256-4201 |
| clinical@medcompnet.com | |
| Phone | +1(215)256-4201 |
| clinical@medcompnet.com | |
| Phone | +1(215)256-4201 |
| clinical@medcompnet.com | |
| Phone | +1(215)256-4201 |
| clinical@medcompnet.com | |
| Phone | +1(215)256-4201 |
| clinical@medcompnet.com | |
| Phone | +1(215)256-4201 |
| clinical@medcompnet.com |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00884908155983 [Primary] |
| GS1 | 50884908155988 [Package] Contains: 00884908155983 Package: box [5 Units] In Commercial Distribution |
FDA Product Code
| LJS | Catheter, intravascular, therapeutic, long-term greater than 30 days |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | true |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2023-05-31 |
| Device Publish Date | 2023-05-23 |
On-Brand Devices [PRO-LINE® CT PRESSURE INJECTABLE CVC (ISOPLAST)]
| 50884908172992 | 5F X 60CM SINGLE LUMEN PRO-LINE® CT BASIC IR SET |
| 50884908172985 | 6F X 60CM SINGLE LUMEN PRO-LINE® CT BASIC IR SET |
| 50884908172961 | 5F X 55CM DUAL LUMEN PRO-LINE® CT W/CUFF @ 5CM BASIC IR SET |
| 50884908155988 | 6F X 60CM DUAL LUMEN PRO-LINE® CT W/CUFF @ 5CM BASIC IR SET |
| 50884908140618 | 7F X 60CM SINGLE LUMEN PRO-LINE® CT BASIC IR SET |
| 50884908140601 | 6F X 60CM TRIPLE LUMEN PRO-LINE® CT BASIC IR SET |
| 50884908140465 | 7F X 60CM DUAL LUMEN PRO-LINE® CT BASIC IR SET |
| 50884908180515 | 6F X 60CM SINGLE LUMEN PRO-LINE® CT BASIC IR SET |
| 50884908180645 | 7F X 60CM SINGLE LUMEN PRO-LINE® CT BASIC IR SET |
| 50884908180539 | 6F X 60CM DUAL LUMEN PRO-LINE® CT W/CUFF @ 5CM BASIC IR SET |
| 50884908180492 | 5F X 55CM DUAL LUMEN PRO-LINE® CT W/CUFF @ 5CM BASIC IR SET |
| 50884908180478 | 5F X 60CM SINGLE LUMEN PRO-LINE® CT BASIC IR SET |
| 50884908135614 | 5F X 55CM DUAL LUMEN PRO-LINE® CT W/CUFF @ 2CM BASIC IR SET |
| 50884908135607 | 5F X 55CM DUAL LUMEN PRO-LINE® CT W/CUFF @ 5CM BASIC IR SET |
| 50884908143862 | 7F X 60CM SINGLE LUMEN PRO-LINE® CT BASIC IR SET |
| 50884908143855 | 7F X 60CM DUAL LUMEN PRO-LINE® CT BASIC IR SET |
| 50884908135782 | 6F X 60CM TRIPLE LUMEN PRO-LINE® CT BASIC IR SET |
Trademark Results [PRO-LINE]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
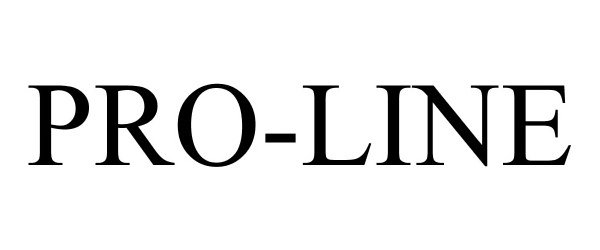 PRO-LINE 87886047 5637047 Live/Registered |
D.T. Mattson Enterprises, Inc. 2018-04-20 |
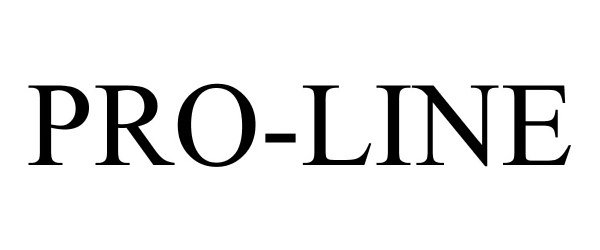 PRO-LINE 86482307 5020095 Live/Registered |
CQT KENNEDY, LLC 2014-12-16 |
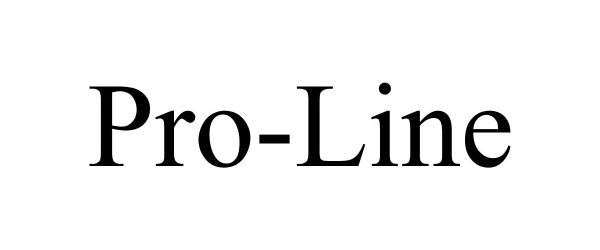 PRO-LINE 85802453 4419898 Live/Registered |
elite grips USA Inc. 2012-12-13 |
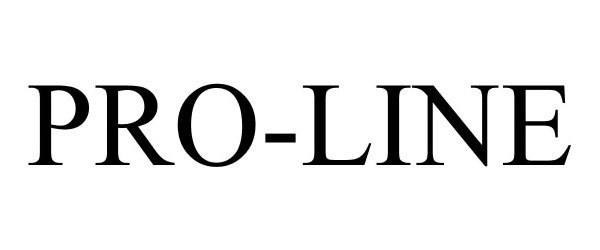 PRO-LINE 85776109 4361290 Live/Registered |
Pro Line Distributing, Inc. 2012-11-09 |
 PRO-LINE 85122256 3957361 Dead/Cancelled |
D.T. MATTSON ENTERPRISES, INC. 2010-09-02 |
 PRO-LINE 78171523 not registered Dead/Abandoned |
Pro-Line International, Inc. 2002-10-07 |
 PRO-LINE 77284233 3504002 Live/Registered |
Pro-Line Performance, Inc. 2007-09-20 |
 PRO-LINE 76667309 3521029 Dead/Cancelled |
Medical Components, Inc. 2006-10-12 |
 PRO-LINE 76522806 not registered Dead/Abandoned |
DT MATTSON ENTERPRISES, INC. 2003-06-16 |
 PRO-LINE 76373872 2744495 Live/Registered |
LA-CO INDUSTRIES, INC. 2002-02-21 |
 PRO-LINE 76336366 2731351 Dead/Cancelled |
Mulholland Headwear, Ltd. 2001-11-13 |
 PRO-LINE 76277373 3097018 Dead/Cancelled |
L & P Property Management Company 2001-06-27 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.