DEJA VU 24000-02W
GUDID 50885380179660
Cardinal Health Vessel Loops Maxi White
Cardinal Health 200, LLC
Surgical vessel loop| Primary Device ID | 50885380179660 |
| NIH Device Record Key | 92c70ff0-ed25-4def-b1f8-ba64c3a639b7 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | DEJA VU |
| Version Model Number | 24000-02W |
| Catalog Number | 24000-02W |
| Company DUNS | 961027315 |
| Company Name | Cardinal Health 200, LLC |
| Device Count | 2 |
| DM Exempt | false |
| Pre-market Exempt | true |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | true |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 10885380179662 [Unit of Use] |
| GS1 | 20885380179669 [Primary] |
| GS1 | 30885380179666 [Package] Contains: 20885380179669 Package: BOX [10 Units] In Commercial Distribution |
| GS1 | 50885380179660 [Package] Contains: 30885380179666 Package: CASE [10 Units] In Commercial Distribution |
FDA Product Code
| KDC | INSTRUMENT, SURGICAL, DISPOSABLE |
Sterilization
| Steralize Prior To Use | true |
| Device Is Sterile | true |
[50885380179660]
Ethylene Oxide
[50885380179660]
Ethylene Oxide
[50885380179660]
Ethylene Oxide
[50885380179660]
Ethylene Oxide
[50885380179660]
Ethylene Oxide
[50885380179660]
Ethylene Oxide
[50885380179660]
Ethylene Oxide
[50885380179660]
Ethylene Oxide
[50885380179660]
Ethylene Oxide
[50885380179660]
Ethylene Oxide
[50885380179660]
Ethylene Oxide
[50885380179660]
Ethylene Oxide
[50885380179660]
Ethylene Oxide
[50885380179660]
Ethylene Oxide
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2021-05-04 |
| Device Publish Date | 2021-04-26 |
On-Brand Devices [DEJA VU]
| 10885380180187 | Maxi Yellow Vessel Loops, Bulk Non-Sterile, 1000EA/CS |
| 50885380179660 | Cardinal Health Vessel Loops Maxi White |
Trademark Results [DEJA VU]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 DEJA VU 98588986 not registered Live/Pending |
Now That's TV, LLC 2024-06-06 |
 DEJA VU 97565716 not registered Live/Pending |
Kuykendall, David L II 2022-08-25 |
 DEJA VU 90067165 not registered Live/Pending |
Deja Vu Hair Studio, Corp 2020-07-22 |
 DEJA VU 86965924 not registered Dead/Abandoned |
Guang Zhou Match Garments Co. Ltd 2016-04-06 |
 DEJA VU 86965907 not registered Dead/Abandoned |
Guang Zhou Match Garments Co. Ltd 2016-04-06 |
 DEJA VU 86728627 not registered Dead/Abandoned |
INSTASIZE, INC. 2015-08-18 |
 DEJA VU 86535918 5389060 Live/Registered |
Deja Vu 2015-02-16 |
 DEJA VU 86035654 4677318 Live/Registered |
FEMICA INC 2013-08-12 |
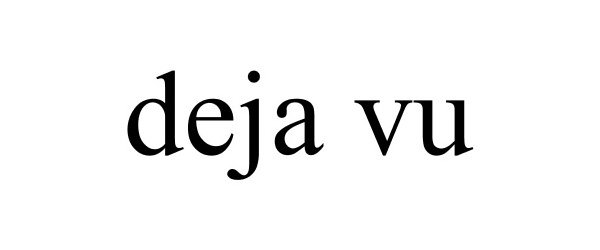 DEJA VU 85370371 not registered Dead/Abandoned |
Glaser, Jan 2011-07-13 |
 DEJA VU 85216627 not registered Dead/Abandoned |
ROYAL WHOLESALE, LLC 2011-01-13 |
 DEJA VU 85099465 not registered Dead/Abandoned |
Smith & Hook Winery, Inc. 2010-08-03 |
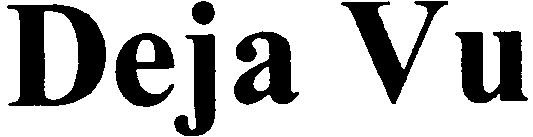 DEJA VU 79096931 not registered Dead/Abandoned |
Brigitte Gertz-Ziegler 2011-04-08 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.