Medichoice
GUDID 50885632134355
Gel Ultrasound Standard 8 Ounce Non-Sterile Fragrance Free Glacial Tint Not Made With Natural Rubber Latex MediChoice
OWENS & MINOR DISTRIBUTION, INC.
Topical coupling gel, non-sterile| Primary Device ID | 50885632134355 |
| NIH Device Record Key | 802d5098-eba6-4170-974c-b6765c12ddd0 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | Medichoice |
| Version Model Number | M500812 |
| Company DUNS | 007941230 |
| Company Name | OWENS & MINOR DISTRIBUTION, INC. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | true |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Operating and Storage Conditions
| Special Storage Condition, Specify | Between 0 and 0 *Avoid Direct Sunlight |
| Handling Environment Temperature | Between -4 Degrees Fahrenheit and 104 Degrees Fahrenheit |
| Special Storage Condition, Specify | Between 0 and 0 |
| Special Storage Condition, Specify | Between 0 and 0 *Avoid Direct Sunlight |
| Handling Environment Temperature | Between -4 Degrees Fahrenheit and 104 Degrees Fahrenheit |
| Special Storage Condition, Specify | Between 0 and 0 |
| Special Storage Condition, Specify | Between 0 and 0 *Avoid Direct Sunlight |
| Handling Environment Temperature | Between -4 Degrees Fahrenheit and 104 Degrees Fahrenheit |
| Special Storage Condition, Specify | Between 0 and 0 |
| Special Storage Condition, Specify | Between 0 and 0 *Avoid Direct Sunlight |
| Handling Environment Temperature | Between -4 Degrees Fahrenheit and 104 Degrees Fahrenheit |
| Special Storage Condition, Specify | Between 0 and 0 |
| Special Storage Condition, Specify | Between 0 and 0 *Avoid Direct Sunlight |
| Handling Environment Temperature | Between -4 Degrees Fahrenheit and 104 Degrees Fahrenheit |
| Special Storage Condition, Specify | Between 0 and 0 |
| Special Storage Condition, Specify | Between 0 and 0 *Avoid Direct Sunlight |
| Handling Environment Temperature | Between -4 Degrees Fahrenheit and 104 Degrees Fahrenheit |
| Special Storage Condition, Specify | Between 0 and 0 |
| Special Storage Condition, Specify | Between 0 and 0 *Avoid Direct Sunlight |
| Handling Environment Temperature | Between -4 Degrees Fahrenheit and 104 Degrees Fahrenheit |
| Special Storage Condition, Specify | Between 0 and 0 |
| Special Storage Condition, Specify | Between 0 and 0 *Avoid Direct Sunlight |
| Handling Environment Temperature | Between -4 Degrees Fahrenheit and 104 Degrees Fahrenheit |
| Special Storage Condition, Specify | Between 0 and 0 |
| Special Storage Condition, Specify | Between 0 and 0 *Avoid Direct Sunlight |
| Handling Environment Temperature | Between -4 Degrees Fahrenheit and 104 Degrees Fahrenheit |
| Special Storage Condition, Specify | Between 0 and 0 |
| Special Storage Condition, Specify | Between 0 and 0 *Avoid Direct Sunlight |
| Handling Environment Temperature | Between -4 Degrees Fahrenheit and 104 Degrees Fahrenheit |
| Special Storage Condition, Specify | Between 0 and 0 |
| Special Storage Condition, Specify | Between 0 and 0 *Avoid Direct Sunlight |
| Handling Environment Temperature | Between -4 Degrees Fahrenheit and 104 Degrees Fahrenheit |
| Special Storage Condition, Specify | Between 0 and 0 |
| Special Storage Condition, Specify | Between 0 and 0 *Avoid Direct Sunlight |
| Handling Environment Temperature | Between -4 Degrees Fahrenheit and 104 Degrees Fahrenheit |
| Special Storage Condition, Specify | Between 0 and 0 |
| Special Storage Condition, Specify | Between 0 and 0 *Avoid Direct Sunlight |
| Handling Environment Temperature | Between -4 Degrees Fahrenheit and 104 Degrees Fahrenheit |
| Special Storage Condition, Specify | Between 0 and 0 |
| Special Storage Condition, Specify | Between 0 and 0 *Avoid Direct Sunlight |
| Handling Environment Temperature | Between -4 Degrees Fahrenheit and 104 Degrees Fahrenheit |
| Special Storage Condition, Specify | Between 0 and 0 |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 10885632134357 [Primary] |
| GS1 | 50885632134355 [Package] Contains: 10885632134357 Package: [12 Units] In Commercial Distribution |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K955246
FDA Product Code
| MUI | Media,coupling,ultrasound |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 4 |
| Public Version Date | 2022-06-10 |
| Device Publish Date | 2016-08-29 |
On-Brand Devices [Medichoice]
| 50885632135284 | Adapter Blood Pressure Cuff X 2-Tube Female Subminiature For Marquette Welch Allyn Lxi Cvsm Moni |
| 50885632131620 | Adapter Blood Pressure Cuff 1-Tube Female Subminiature To 1-Tube Bayonet Cuff For Propaq Not Mad |
| 50885632131613 | Adapter Blood Pressure Cuff Y 2-Tube Male And Female Subminiature To 1-Tube Bayonet Cuff For Dat |
| 50885632131606 | Adapter Blood Pressure Cuff X 2-Tube Bulb And Valve To 2-Tube Screw Cuff For Manual Sphygmomanom |
| 50885632131590 | Adapter Blood Pressure Cuff Y 1-Tube Screw To 2-Tube Screw Cuff For Welch Allyn Spot Vsm Monitor |
| 50885632131576 | Adapter Blood Pressure Cuff Y 1-Tube Luer Lock To 2-Tube Screw Cuff For Datascope Siemens Monito |
| 50885632115958 | Adapter Blood Pressure Cuff 1-Tube Screw To 1-Tube Bayonet Cuff For Welch Allyn Spot Vsm Monitor |
| 50885632115941 | Adapter Blood Pressure Cuff Y 2-Tube Female Subminiature To 1-Tube Bayonet Cuff For Marquette We |
| 50885632115934 | Adapter Blood Pressure Cuff Y 2-Tube Screw To 1-Tube Bayonet Cuff For Dinamap Monitor Not Made W |
| 50885632115927 | Adapter Blood Pressure Cuff Y 2-Tube Bulb And Valve To 1-Tube Bayonet Cuff For Manual Sphygmoman |
| 50885632115910 | Adapter Blood Pressure Cuff Y 1-Tube Male Bayonet To 2-Tube Screw Cuff For Philips Hp Agilent Da |
| 50885632115903 | Adapter Blood Pressure Cuff X 2-Tube Male And Female Subminiature To 2-Tube Screw Cuff For Datex |
| 50885632138247 | Gown Isolation Aami Level 3 Sms Full Back Overhead Thumbloop With Elastic Cuff Tie Waist Blue Xl |
| 50885632138230 | Gown Isolation Aami Level 3 Sms Full Back Overhead Thumbloop With Elastic Cuff Tie Waist Blue Un |
| 50885632138223 | Gown Isolation Aami Level 3 Sms Full Back Overhead Thumbloop With Elastic Cuff Tie Waist Yellow |
| 50885632138216 | Gown Isolation Isolation AAMI Level 3 SMS Full Back Overhead Thumbloop With Elastic Cuff Tie Wai |
| 50885632135628 | Gown Isolation Procedure Aami Level 3 Sms Full Back Knit Cuff Hook And Loop Neck Tie Waist Blue |
| 50885632135611 | Gown Isolation Procedure Aami Level 3 Sms Full Back Knit Cuff Hook And Loop Neck Closure Waist B |
| 50885632133389 | Gel Ultrasound Premium 20 Gram Non-Sterile Single Patient Packet Fragrance Free Clear Not Made W |
| 50885632127647 | Solution Respiratory 9 Percent Sodium Chloride Convenient Twist-Off Cap 15 Milliliter Sterile No |
| 50885632127630 | Solution Respiratory 9 Percent Sodium Chloride Convenient Twist-Off Cap 5 Milliliter Sterile Not |
| 50885632127623 | FDA Class N/A Import Latex Free Physician Formulary Sterile |
| 50885632100305 | Gown Isolation Aami Level 3 Sms Full Back Elastic Cuff Tie Neck And Waist Yellow Xl Not Made Wit |
| 50885632100046 | Gown Isolation Aami Level 3 Sms Full Back Elastic Cuff Tie Neck And Waist Yellow Universal Not M |
| 20885632066617 | Mask Procedure Face PRIMAGARD80 Isolation with Extended Loop Yellow Not Made With Natural Rubber |
| 50885632115859 | Cuff Blood Pressure Bladder Single Patient Use Soft-fabric 2-Tube With Female Subminiature-Locki |
| 50885632115842 | Cuff Blood Pressure Bladder Single Patient Use Soft-fabric 2-Tube With Female Subminiature-Locki |
| 50885632115835 | Cuff Blood Pressure Bladder Single Patient Use Soft-fabric 1-Tube With Bayonet-Rectus-HP Connect |
| 50885632115828 | Cuff Blood Pressure Bladder Single Patient Use Soft-fabric 2-Tube With Female Subminiature-Locki |
| 50885632115811 | Cuff Blood Pressure Bladder Single Patient Use Soft-fabric 2-Tube With Female Subminiature-Locki |
| 50885632115804 | Cuff Blood Pressure Bladder Single Patient Use Soft-fabric 1-Tube With Bayonet-Rectus-HP Connect |
| 50885632115798 | Cuff Blood Pressure Bladder Single Patient Use Soft-fabric 1-Tube With Screw Connector Adult Lon |
| 50885632115781 | Cuff Blood Pressure Bladder Single Patient Use Soft-fabric 1-Tube With Screw Connector Small Adu |
| 50885632115774 | Cuff Blood Pressure Bladder Single Patient Use Soft-fabric 1-Tube With Screw Connector Child Not |
| 50885632115767 | Cuff Blood Pressure Bladder Single Patient Use Soft-fabric 1-Tube With Bayonet-Rectus-HP Connect |
| 50885632115750 | Cuff Blood Pressure Bladder Single Patient Use Soft-fabric 2-Tube With Female Subminiature-Locki |
| 50885632115743 | Cuff Blood Pressure Bladder Single Patient Use Soft-fabric 1-Tube With Screw Connector Large Adu |
| 50885632115736 | Cuff Blood Pressure Bladder Single Patient Use Soft-fabric 1-Tube With Screw Connector Large Adu |
| 50885632115729 | Cuff Blood Pressure Bladder Single Patient Use Soft-fabric 1-Tube With Screw Connector Adult Not |
| 50885632115712 | Cuff Blood Pressure Bladder Single Patient Use Soft-fabric 2-Tube With Screw Connector Large Adu |
| 50885632115705 | Cuff Blood Pressure Bladder Single Patient Use Soft-fabric 1-Tube With Bayonet-Rectus-HP Connect |
| 50885632115699 | Cuff Blood Pressure Bladder Single Patient Use Soft-fabric 2-Tube With Screw Connector Adult Lon |
| 50885632115682 | Cuff Blood Pressure Bladder Single Patient Use Soft-fabric 1-Tube With Bayonet-Rectus-HP Connect |
| 50885632115675 | Cuff Blood Pressure Bladder Single Patient Use Soft-fabric 2-Tube With Screw Connector Adult Not |
| 50885632115668 | Cuff Blood Pressure Bladder Single Patient Use Soft-fabric 1-Tube With Bayonet-Rectus-HP Connect |
| 50885632115651 | Cuff Blood Pressure Bladder Single Patient Use Soft-fabric 2-Tube With Female Subminiature-Locki |
| 50885632115644 | Cuff Blood Pressure Bladder Single Patient Use Soft-fabric 2-Tube With Screw Connector Large Adu |
| 50885632115637 | Cuff Blood Pressure Bladder Single Patient Use Soft-fabric 2-Tube With Screw Connector Small Adu |
| 50885632115620 | Cuff Blood Pressure Bladder Single Patient Use Soft-fabric 2-Tube With Screw Connector Child Not |
| 50885632115552 | Cuff Blood Pressure Bladder Inflation System Nylon Reusable 2-Tube With Bulb And Valve Child Not |
Trademark Results [Medichoice]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 MEDICHOICE 78570543 not registered Dead/Abandoned |
Mount Carmel Health Plan, Inc. 2005-02-18 |
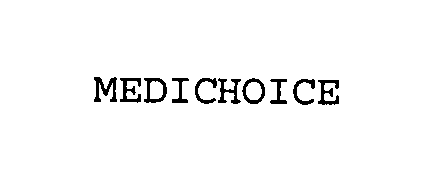 MEDICHOICE 76978548 3283434 Live/Registered |
Owens & Minor, Inc. 2001-12-26 |
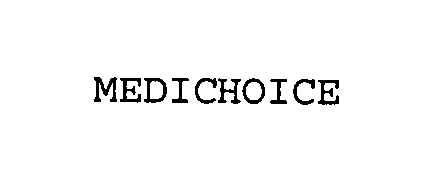 MEDICHOICE 76352417 3462755 Live/Registered |
Owens & Minor, Inc. 2001-12-26 |
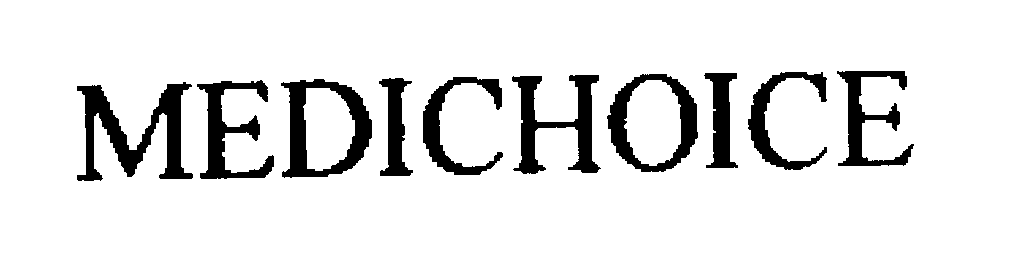 MEDICHOICE 76285309 2753896 Live/Registered |
Owens & Minor, Inc. 2001-07-13 |
 MEDICHOICE 75267174 not registered Dead/Abandoned |
Blue Ridge Health Alliance 1997-04-01 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.