EASYGRIP FLO-41 ADS201897
GUDID 55413765586113
The EASYGRIP FLO-41 Precision MIS Delivery System (EASYGRIP FLO-41 System) is a sterile, single-use device that consists of two components: (1) one applicator device with a 41 cm long cannula (5 mm outer diameter) and (2) one empty 1.5 mL syringe.
BAXTER HEALTHCARE CORPORATION
Haematological concentrate applicator| Primary Device ID | 55413765586113 |
| NIH Device Record Key | 51a80c6a-390c-4bb1-affd-7de4c2d5b4b0 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | EASYGRIP FLO-41 |
| Version Model Number | ADS201897 |
| Catalog Number | ADS201897 |
| Company DUNS | 005083209 |
| Company Name | BAXTER HEALTHCARE CORPORATION |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | true |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | true |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
Device Dimensions
| Length | 41 Centimeter |
| Length | 41 Centimeter |
| Length | 41 Centimeter |
| Length | 41 Centimeter |
| Length | 41 Centimeter |
| Length | 41 Centimeter |
| Length | 41 Centimeter |
| Length | 41 Centimeter |
| Length | 41 Centimeter |
| Length | 41 Centimeter |
| Length | 41 Centimeter |
| Length | 41 Centimeter |
| Length | 41 Centimeter |
| Length | 41 Centimeter |
| Length | 41 Centimeter |
| Length | 41 Centimeter |
| Length | 41 Centimeter |
| Length | 41 Centimeter |
| Length | 41 Centimeter |
| Length | 41 Centimeter |
| Length | 41 Centimeter |
| Length | 41 Centimeter |
| Length | 41 Centimeter |
| Length | 41 Centimeter |
| Length | 41 Centimeter |
| Length | 41 Centimeter |
| Length | 41 Centimeter |
| Length | 41 Centimeter |
| Length | 41 Centimeter |
| Length | 41 Centimeter |
| Length | 41 Centimeter |
| Length | 41 Centimeter |
| Length | 41 Centimeter |
| Length | 41 Centimeter |
| Length | 41 Centimeter |
| Length | 41 Centimeter |
| Length | 41 Centimeter |
| Length | 41 Centimeter |
| Length | 41 Centimeter |
| Length | 41 Centimeter |
| Length | 41 Centimeter |
| Length | 41 Centimeter |
| Length | 41 Centimeter |
| Length | 41 Centimeter |
| Length | 41 Centimeter |
| Length | 41 Centimeter |
| Length | 41 Centimeter |
| Length | 41 Centimeter |
| Length | 41 Centimeter |
Operating and Storage Conditions
| Storage Environment Temperature | Between 2 Degrees Celsius and 25 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 25 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 25 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 25 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 25 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 25 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 25 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 25 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 25 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 25 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 25 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 25 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 25 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 25 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 25 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 25 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 25 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 25 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 25 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 25 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 25 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 25 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 25 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 25 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 25 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 25 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 25 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 25 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 25 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 25 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 25 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 25 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 25 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 25 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 25 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 25 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 25 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 25 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 25 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 25 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 25 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 25 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 25 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 25 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 25 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 25 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 25 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 25 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 25 Degrees Celsius |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 05413765586118 [Primary] |
| GS1 | 55413765586113 [Package] Contains: 05413765586118 Package: CASE [6 Units] In Commercial Distribution |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K193137
FDA Product Code
| GCJ | Laparoscope, general & plastic surgery |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | true |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2020-04-16 |
| Device Publish Date | 2020-04-08 |
Devices Manufactured by BAXTER HEALTHCARE CORPORATION
| 05413765580970 - RECOTHROM SPRAY KIT | 2025-12-09 RECOTHROM SPRAY APPLICATOR KIT includes several components designed for topical application of Recothrom onto the bleeding site |
| 00085412002750 - DUO-VENT | 2025-10-06 Nitroglycerin Set with DUO-VENT Spike, PVC Tubing Segment, Injection Site, Male Luer Lock Adapter |
| 50197886011239 - Novum IQ | 2025-09-02 The Novum IQ Large Volume Pump (LVP) is a large volume infusion pump system that provides delivery of fluids into a patient in a |
| 50197886013172 - Novum IQ | 2025-09-02 The Novum IQ Large Volume Pump (LVP) is a large volume infusion pump system that provides delivery of fluids into a patient in a |
| 50197886013189 - Novum IQ | 2025-09-02 The Novum IQ Large Volume Pump (LVP) is a large volume infusion pump system that provides delivery of fluids into a patient in a |
| 50197886013196 - Novum IQ | 2025-09-02 The Novum IQ Large Volume Pump (LVP) is a large volume infusion pump system that provides delivery of fluids into a patient in a |
| 20197886002304 - Novum IQ | 2025-07-15 The Novum IQ Syringe Pump is software controlled Syringe infusion pump system that provides delivery of fluids into a patient in |
| 20197886008207 - Novum IQ | 2025-07-15 The Novum IQ Syringe Pump is software controlled Syringe infusion pump system that provides delivery of fluids into a patient in |
Trademark Results [EASYGRIP FLO-41]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
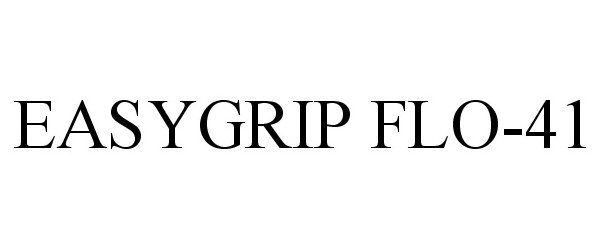 EASYGRIP FLO-41 88181951 not registered Live/Pending |
Baxter International Inc. 2018-11-05 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.