Mswab® 404C
GUDID 58053326006185
1,6 mL of MSwab® medium + regular FLOQSwabs®
COPAN ITALIA SPA
General specimen collection kit| Primary Device ID | 58053326006185 |
| NIH Device Record Key | 3a0bb95c-163c-4aaf-994c-1c27486f33ed |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | Mswab® |
| Version Model Number | 404C |
| Catalog Number | 404C |
| Company DUNS | 428981112 |
| Company Name | COPAN ITALIA SPA |
| Device Count | 50 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 18053326006187 [Unit of Use] |
| GS1 | 38053326006181 [Primary] |
| GS1 | 58053326006185 [Package] Contains: 38053326006181 Package: [6 Units] In Commercial Distribution |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K121039
FDA Product Code
| JTW | System, Transport, Aerobic |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2022-12-15 |
| Device Publish Date | 2022-12-07 |
On-Brand Devices [Mswab® ]
| 58053326006192 | MSwab Kit 1,6 mL Collection transport and preservation system for Gram positive aerobic & facult |
| 58053326006185 | 1,6 mL of MSwab® medium + regular FLOQSwabs® |
Trademark Results [Mswab]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
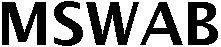 MSWAB 79152720 4843309 Live/Registered |
COPAN ITALIA S.P.A. 2014-03-31 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.