BIOLIGHT
GUDID 66714700001330
BIOLIGHT DUAL REAMER #3
Synca Marketing Inc
Fluted surgical drill bit, reusable| Primary Device ID | 66714700001330 |
| NIH Device Record Key | a5b9c017-6e42-49d7-a6c8-be3ce9f5b2f6 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | BIOLIGHT |
| Version Model Number | BDT33 |
| Company DUNS | 247111768 |
| Company Name | Synca Marketing Inc |
| Device Count | 1 |
| DM Exempt | true |
| Pre-market Exempt | true |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | false |
| OTC Over-The-Counter | false |
Customer Support Contacts
| Phone | 1-800-667-9622 |
| XXX@XX.XX | |
| Phone | 1-800-667-9622 |
| XXX@XX.XX | |
| Phone | 1-800-667-9622 |
| XXX@XX.XX | |
| Phone | 1-800-667-9622 |
| XXX@XX.XX | |
| Phone | 1-800-667-9622 |
| XXX@XX.XX | |
| Phone | 1-800-667-9622 |
| XXX@XX.XX | |
| Phone | 1-800-667-9622 |
| XXX@XX.XX | |
| Phone | 1-800-667-9622 |
| XXX@XX.XX | |
| Phone | 1-800-667-9622 |
| XXX@XX.XX | |
| Phone | 1-800-667-9622 |
| XXX@XX.XX | |
| Phone | 1-800-667-9622 |
| XXX@XX.XX | |
| Phone | 1-800-667-9622 |
| XXX@XX.XX | |
| Phone | 1-800-667-9622 |
| XXX@XX.XX | |
| Phone | 1-800-667-9622 |
| XXX@XX.XX | |
| Phone | 1-800-667-9622 |
| XXX@XX.XX | |
| Phone | 1-800-667-9622 |
| XXX@XX.XX | |
| Phone | 1-800-667-9622 |
| XXX@XX.XX | |
| Phone | 1-800-667-9622 |
| XXX@XX.XX | |
| Phone | 1-800-667-9622 |
| XXX@XX.XX |
Device Dimensions
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 66714700001330 [Primary] |
FDA Product Code
| ELR | Post, Root Canal |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 3 |
| Public Version Date | 2018-11-22 |
| Device Publish Date | 2016-09-27 |
On-Brand Devices [BIOLIGHT]
| 66714700001453 | BIOLIGHT ST EPOXY-FREE FIBER POSTS INTRO KIT |
| 66714700001439 | BIOLIGHT ST REAMER 1.50 MM |
| 66714700001422 | BIOLIGHT ST REAMER 1.35 MM |
| 66714700001415 | BIOLIGHT ST REAMER 1.20 MM |
| 66714700001408 | BIOLIGHT ST REAMER 1.00 MM |
| 66714700001385 | BIOLIGHT ST REFILL 1.50 MM |
| 66714700001378 | BIOLIGHT ST REFILL 1.35 MM |
| 66714700001361 | BIOLIGHT ST REFILL 1.20 MM |
| 66714700001354 | BIOLIGHT ST REFILL 1.00 MM |
| 66714700001347 | BIOLIGHT DUAL ADVANCED FIBER POSTS INTRO KIT |
| 66714700001330 | BIOLIGHT DUAL REAMER #3 |
| 66714700001323 | BIOLIGHT DUAL REAMER #2 |
| 66714700001316 | BIOLIGHT DUAL REAMER #1 |
| 66714700001309 | BIOLIGHT DUAL REAMER #0.5 |
| 66714700001293 | BIOLIGHT DUAL REFILL #3 |
| 66714700001286 | BIOLIGHT DUAL REFILL #2 |
| 66714700001279 | BIOLIGHT DUAL REFILL #1 |
| 66714700001262 | BIOLIGHT DUAL REFILL #0.5 |
| 66714700000159 | BIOLIGHT TEMP CROWN & BRIDGE A2 |
| 00667147001309 | BIOLIGHT DUAL REAMER #0.5 |
| 00667147000449 | BIOLIGHT ST EPOXY-FREE FIBER POSTS INTRO KIT |
| 00667147000425 | BIOLIGHT ST REAMER 1.50 MM |
| 00667147000418 | BIOLIGHT ST REAMER 1.35 MM |
| 00667147000401 | BIOLIGHT ST REAMER 1.20 MM |
| 00667147000395 | BIOLIGHT ST REAMER 1.00 MM |
| 00667147000333 | BIOLIGHT DUAL ADVANCED FIBER POSTS INTRO KIT |
| 00667147000326 | BIOLIGHT DUAL REAMER #3 |
| 00667147000319 | BIOLIGHT DUAL REAMER #2 |
| 00667147000302 | BIOLIGHT DUAL REAMER #1 |
| 00667147000296 | BIOLIGHT DUAL REAMER # 0.5 |
| 00667147100255 | BIOLIGHT DUAL REFILL #0.5 |
| 00667147100378 | BIOLIGHT ST REFILL 1.50 MM |
| 00667147100361 | BIOLIGHT ST REFILL 1.35MM |
| 00667147100354 | BIOLIGHT ST REFILL 1.20MM |
| 00667147100347 | BIOLIGHT ST REFILL 1.00 MM |
| 00667147100286 | BIOLIGHT DUAL REFILL #3 |
| 00667147100279 | BIOLIGHT DUAL REFILL #2 |
| 00667147100262 | BIOLIGHT DUAL REFILL #1 |
| 10667147000446 | BIOLIGHT ST EPOXY-FREE FIBER POSTS INTRO KIT |
| 10667147000422 | BIOLIGHT ST REAMER 1.50 MM |
| 10667147000415 | BIOLIGHT ST REAMER 1.35 MM |
| 10667147000408 | BIOLIGHT ST REAMER 1.20 MM |
| 10667147000392 | BIOLIGHT ST REAMER 1.00 MM |
| 10667147100375 | BIOLIGHT ST REFILL 1.50 MM |
| 10667147100368 | BIOLIGHT ST REFILL 1.35MM |
| 10667147100351 | BIOLIGHT ST REFILL 1.20MM |
| 10667147100344 | BIOLIGHT ST REFILL 1.00 MM |
| 10667147000330 | BIOLIGHT DUAL ADVANCED FIBER POSTS INTRO KIT |
| 10667147000323 | BIOLIGHT DUAL REAMER #3 |
| 10667147000316 | BIOLIGHT DUAL REAMER #2 |
Trademark Results [BIOLIGHT]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
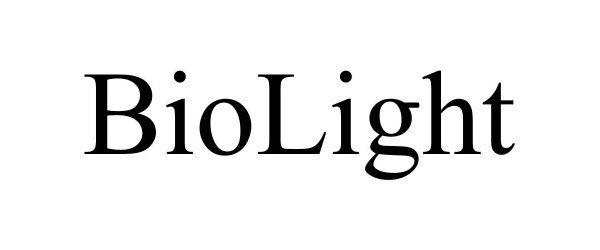 BIOLIGHT 97867724 not registered Live/Pending |
3D Systems, Inc. 2023-03-31 |
 BIOLIGHT 90687742 not registered Live/Pending |
Aqua Science LLC 2021-05-03 |
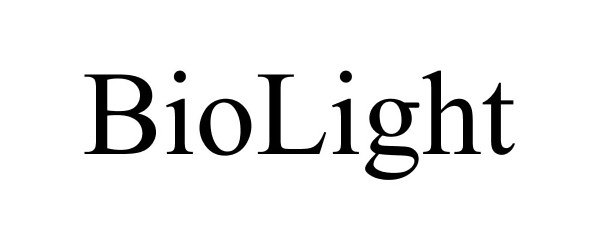 BIOLIGHT 88457474 not registered Live/Pending |
Biolight 2019-06-03 |
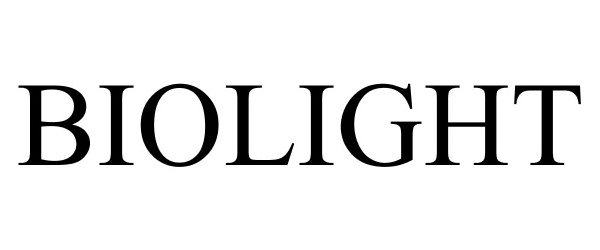 BIOLIGHT 87552669 5412530 Live/Registered |
Sarkli-Repechage, Ltd. 2017-08-02 |
 BIOLIGHT 87532623 not registered Dead/Abandoned |
Jose Fernandez 2017-07-18 |
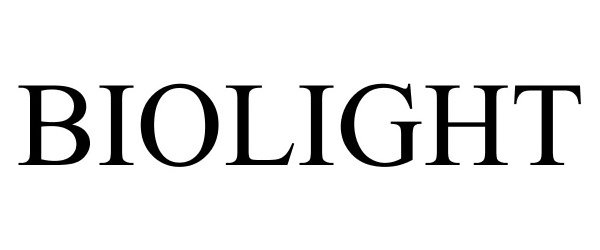 BIOLIGHT 86127920 4694274 Live/Registered |
Synca Marketing Inc 2013-11-25 |
 BIOLIGHT 85811166 4379964 Live/Registered |
Bioscan, Inc. 2012-12-27 |
 BIOLIGHT 85305267 not registered Dead/Abandoned |
Sarkli-Repechage, Ltd. 2011-04-26 |
 BIOLIGHT 77533039 not registered Dead/Abandoned |
Biolight Harvesting, Inc. 2008-07-28 |
 BIOLIGHT 77397367 not registered Dead/Abandoned |
Cyalume Technologies, Inc. 2008-02-14 |
 BIOLIGHT 77325722 not registered Dead/Abandoned |
MARQUEZ BROTHERS INTERNATIONAL, INC. 2007-11-09 |
 BIOLIGHT 75457943 not registered Dead/Abandoned |
BRYAN, BRUCE 1998-03-27 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.